6.6: Enthalpy
Chemists ordinarily use a property known as enthalpy (H) to describe the thermodynamics of chemical and physical processes. Enthalpy is defined as the sum of a system’s internal energy (E) and the mathematical product of its pressure (P) and volume (V):
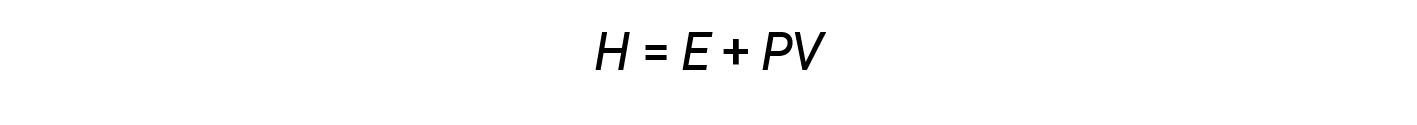
Enthalpy is a state function. Enthalpy values for specific substances cannot be measured directly; only enthalpy changes for chemical or physical processes can be determined. For processes that take place at constant pressure (a common condition for many chemical and physical changes), the enthalpy change (ΔH) is:
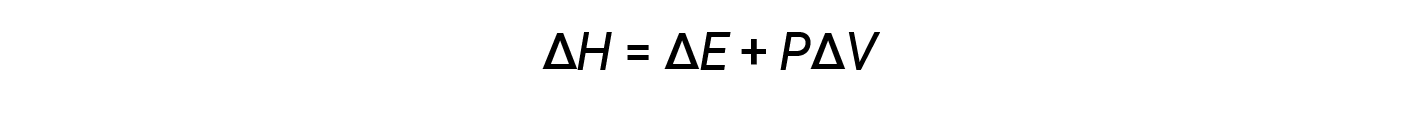
The mathematical product PΔV represents work (w), namely, expansion or pressure-volume work. By their definitions, the arithmetic signs of ΔV and w will always be opposite:
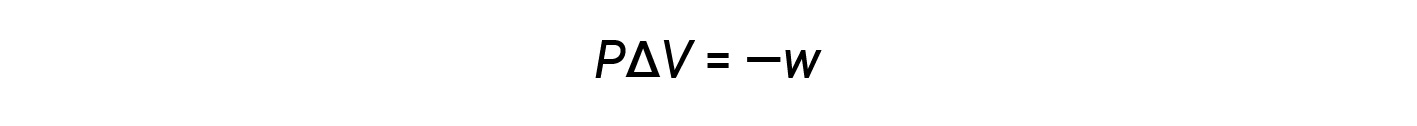
Substituting this equation and the definition of internal energy at constant pressure (ΔE = qp + w) into the enthalpy-change equation yields:
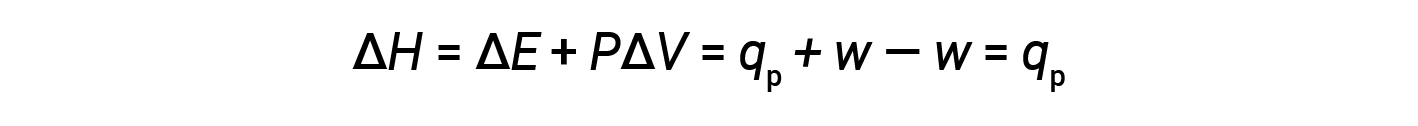
where qp is the heat of reaction under conditions of constant pressure.
And so, if a chemical or physical process is carried out at constant pressure with the only work done caused by expansion or contraction (P-V work), then the heat flow (qp) and enthalpy change (ΔH) for the process are equal.
The heat given off while operating a Bunsen burner is equal to the enthalpy change of the methane combustion reaction that takes place since it occurs at the essentially constant pressure of the atmosphere. Chemists usually perform experiments under normal atmospheric conditions, at constant external pressure with qp = ΔH, which makes enthalpy the most convenient choice for determining heat changes for chemical reactions.
A negative value of an enthalpy change, ΔH < 0, indicates an exothermic reaction (heat given off to the surroundings); a positive value, ΔH > 0, indicates an endothermic reaction (heat absorbed from the surroundings). If the direction of a chemical equation is reversed, the arithmetic sign of its ΔH is changed (a process that is endothermic in one direction is exothermic in the opposite direction).
Conceptually, ΔE (a measure of heat and work) and ΔH (a measure of heat at constant pressure) both represent changes in a state function for the system. In processes where the volume change, ΔV, is small (melting of ice), and ΔE and ΔH are identical. However, if the volume change is significant (evaporation of water), the amount of energy transferred as work will be significant; thus, ΔE and ΔH have significantly different values.
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.


