14.3: التوازن اللتفاعلات الغازية والتفاعلات غير المتجانسة
بالنسبة لتفاعلات الطور الغازي، يمكن التعبير عن ثابت التوازن إما من حيث التركيزات المولية (Kc) أو الضغوط الجزئية (Kp) من المواد المتفاعلة والمنتجات. يمكن اشتقاق العلاقة بين قيمتي K هاتين القيمتين ببساطة من معادلة الغاز المثالية وتعريف المولارية. وفقًا لمعادلة الغاز المثالية:
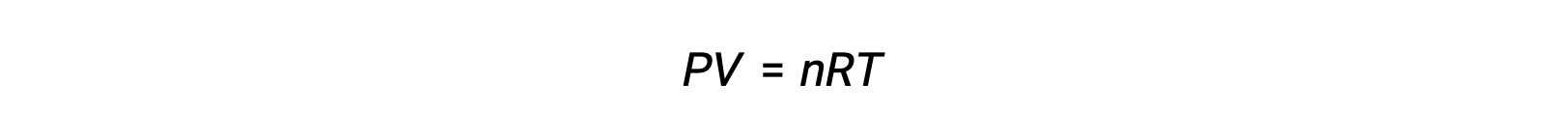
يُعزى التركيز المولي أو المولارية على عدد المولات مقسومًا على الحجم:

فإذاً,
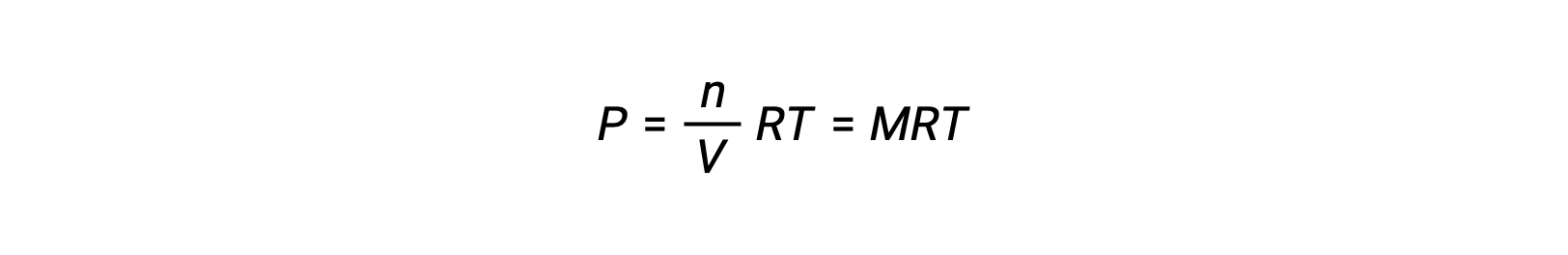
حيث أن P هو الضغط الجزئي، V هو الحجم، n هو عدد المولات، R هو ثابت الغاز، T هو درجة الحرارة، و M هو التركيز المولي.
لتفاعل الطور الغازي: m A + n B ⇌ x C + y D
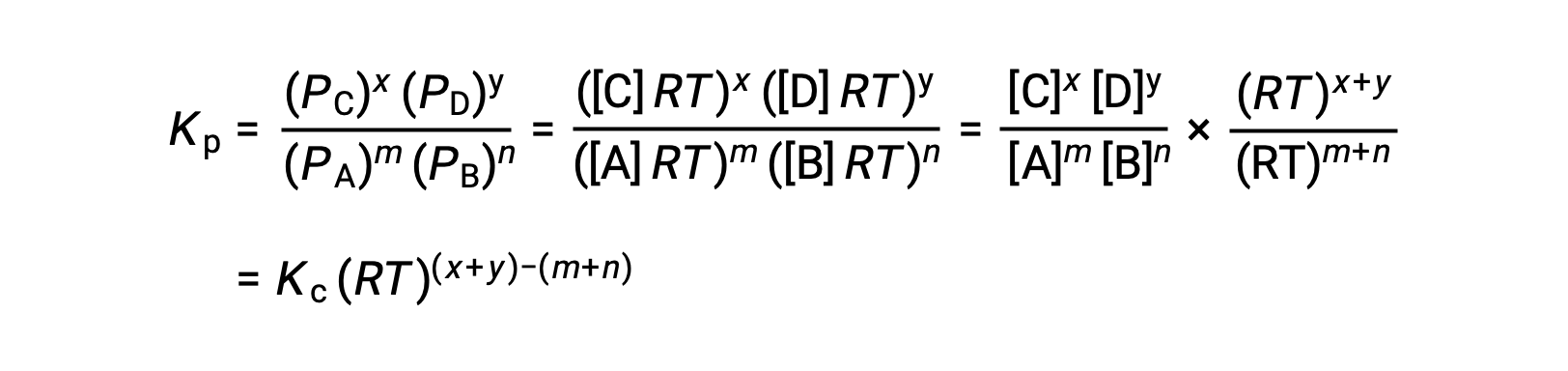
وهكذا، فإن العلاقة بينKc و KP هي
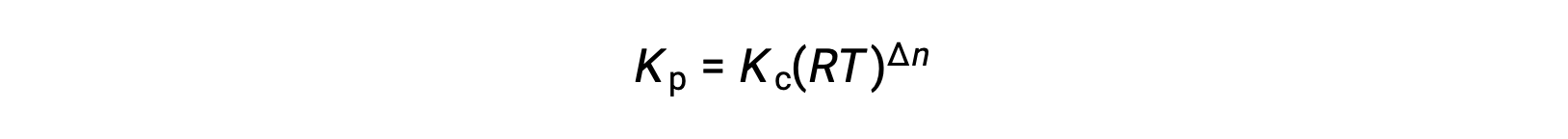
حيث أن Δn هو الفرق في الكميات المولية من المنتج والغازات المتفاعلة، في هذه الحالة:
p style="text-align: center">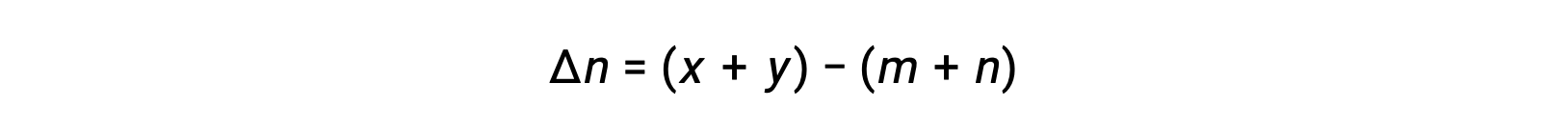
تم اقتباس هذا النص من Openstax, Chemistry 2e, Section 13.2 Equilibrium Constants.


