18.7: Concentration Cells
A concentration cell is a type of a voltaic cell constructed by connecting two almost identical half-cells, both based on the same half-reaction and using the same electrode, differing only in the concentration of one redox species. A concentration cell's potential, therefore, is determined only by the concentration difference of the particular redox species.
Consider the following voltaic cell:
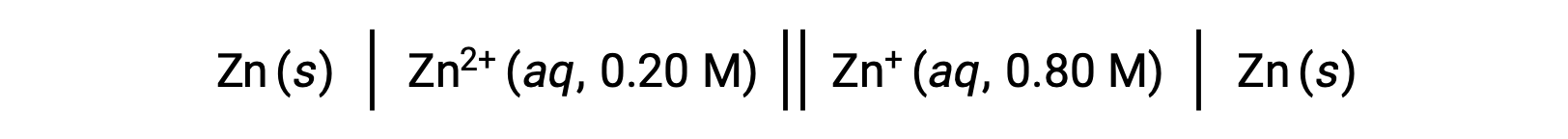
From the given information, the cell potential of this concentration cell can be calculated using the Nernst Equation:
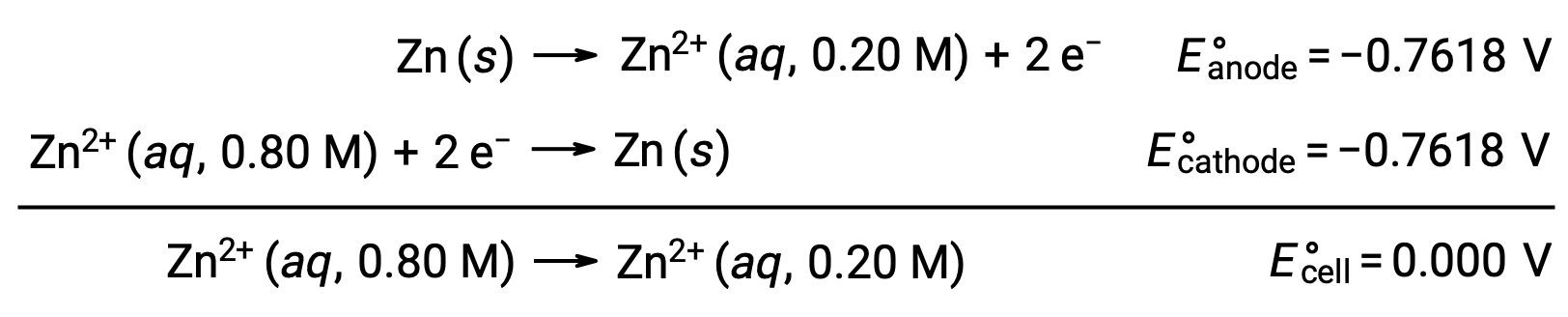
Substituting into the Nernst equation,
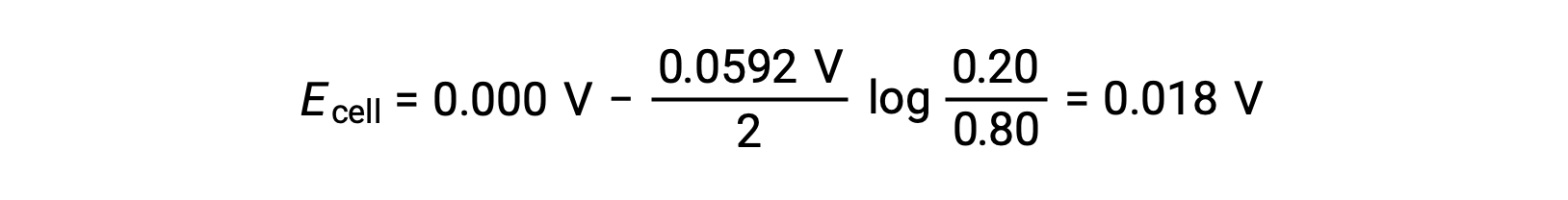
The positive value of the cell potential indicates that the overall cell reaction is spontaneous. This spontaneous reaction occurs when the zinc ion concentration in the cathode falls (by reduction to elemental zinc) while that in the anode rises (by oxidation of the zinc anode to zinc ions). A greater driving force for the reduction of zinc is present in the cathode, where the Zn2+ ion concentration is greater (Ecathode > Eanode).
pH meters in the lab, ion channels in the nerve cell membranes, and cardiac muscle cells in the human body work on the principle of concentration cells.
This text is adapted from Openstax,Chemistry 2e,Chapter 17.4: Potential, Free Energy, and Equilibrium.


