Overview
Source: Laboratory of Jeff Salacup - University of Massachusetts Amherst
The product of an organic solvent extraction, a total lipid extract (TLE), is often a complex mixture of hundreds, if not thousands, of different compounds. The researcher is often only interested in a handful of compounds. The compounds of interest may belong to one of several classes of compounds, such as alkanes, ketones, alcohols, or acids (Figure 1), and it may be useful to remove the compound classes to which it does not belong in order to get a clearer view of the compounds you are interested in. For example, a TLE may contain 1,000 compounds, but the Uk'37 sea surface temperature proxy is based on only two compounds (alkenones) and the TEX86 sea surface temperature proxy is based on only four (glycerol dialkyl glycerol tetraethers). It would behoove the researcher to remove as many of the compounds they are not interested in. This makes the instrumental analysis of the compounds of interest (alkenones or GDGTs) less likely to be complicated by other extraneous compounds.
In other cases, an upstream purification technique may have produced compounds you wish to now remove from the sample, such as the production of carboxylic acids during saponification in our previous video. In both of the above cases the purification technique called column chromatography is very useful.
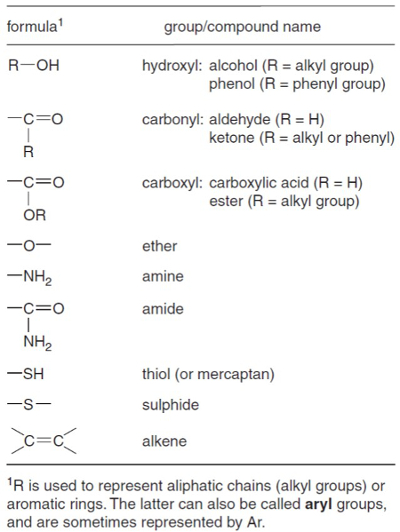
Figure 1. Geochemically important functional groups. From Killops and Killops1.
Principles
Silica gel column chromatography is a purification technique that utilizes the differing associations of discrete compound classes for a silica solid phase, a fine powder called a gel. A small pipette is loaded approximately half full with the gel (Figure 2). This column is then saturated with an apolar solvent, often hexane. A sample is then loaded onto the top of the gel in the column, and a series of solvents of increasing polarity is sequentially passed through the sample in order to separate it into separate compound classes. The separation is based on the affinity of the different compound classes for either the solid phase or the solvent phase. Polar compounds bond more strongly to the silica and therefore take more polar solvents to be washed from the column. Thus, using one column and one sample, that sample can be separated into several fractions; for example, apolars (hydrocarbons), mid-polars (ketones and alcohols), and polars (acids and other functionalized compounds).

Figure 2. Image of a custom-made rack that allows the purification of up to 12 samples at a time.
Column chromatography is a flexible technique for purifying the complex mixture of compounds found in sediment. Mixtures separate as they move through the column and are collected in fractions, each containing a different chemical class of compounds. Therefore, column chromatography is often used as an additional purification step after initial isolation of the desired compound. Organic extracts such as total lipid extracts may be complex mixtures of many compounds. Some purification techniques, such as saponification, introduce compounds that can damage analytical instruments and so must be removed before analysis. This video is part of a series on lipid extraction, purification, and analysis from sediments. Once a total lipid extract is collected from a sedimentary sample, column chromatography is used to purify both alkenones and GDGTs, depending on the desired analysis.
In column chromatography, a mixture of chemical compounds is loaded onto a solid stationary phase such as silica gel. A mobile phase such as an organic solvent is then used to elute, or remove, the compounds from the column. The order in which the compounds are eluted depends on the strength of the interactions of the compounds with the silica gel and with the eluent.
The eluate is collected in fractions, each containing different compounds from the mixture. Depending on the properties of the compounds, a single solvent may provide sufficient separation and elute all of the compounds of interest. Otherwise, multiple solvents are used to elute each compound of interest in turn.
Polar compounds, which have an uneven distribution of charge, adsorb strongly to the polar silica gel, whereas apolar compounds adsorb weakly. Polar solvents have greater affinity for silica gel and therefore are more powerful eluents than apolar solvents. Thus, apolar solvents elute only apolar compounds, whereas polar solvents elute both apolar and polar compounds.
When the desired compounds are moderately polar, apolar compounds should be washed off the column with an apolar solvent before a polar solvent is used. To avoid eluting unwanted highly polar compounds such as acids, a polar eluent should not have more eluting power than needed for the most polar desired compound.
Now that you understand the principles of column chromatography, let's go through a procedure for purification of lipid biomarkers from a total lipid extract by silica gel column chromatography.
To remove organic contaminants, combust borosilicate glass pipettes, borosilicate glass vials, and glass wool for 6 h at 550 °C. Once the glassware is ready for use, set up a rack to hold pipettes and vials. Obtain pipette bulbs, clean tweezers, a laboratory spatula, silica gel, hexane, dichloromethane, and methanol. With clean tweezers, place a small tuft of glass wool into the mouth of a pipette. Gently push the glass wool to the bottom of the pipette with the stem of another pipette to form a loose plug. Carefully load silica gel into the pipette until half full. Secure the pipette upright in the rack. Secure a 4-mL borosilicate glass vial below the tip of the pipette for waste collection. In another borosilicate glass vial, suspend up to 10 mg of dry sample from the saponification process in hexane. If the sample sticks to the walls of the vial, sonicate the vial for 5 min. The chromatography procedure can now begin.
To begin the chromatography, wash the silica gel column with 3 volumes of hexane to remove air bubbles and impurities. Then, replace the waste vial with an empty vial for the apolar fraction. With a glass pipette, load the sample onto the column and allow the suspension to soak into the silica gel. Work quickly so the column does not dry out during the procedure. Rinse the sample vial twice with small portions of hexane and transfer each rinse to the column. Continue adding hexane to the column until the collection vial is nearly full. Allow all of the hexane to finish entering the silica gel. Then, exchange the filled vial with an empty vial for the mid-polar fraction. Next, rinse the sample vial with DCM and add it to the column 3 times. Continue adding DCM to the column until the collection vial is nearly full. Allow the DCM to finish soaking into the silica gel and then exchange the filled vial with an empty vial for the polar fraction. Repeat this process with methanol.
Once the vial is nearly full, allow the methanol to finish dripping into the vial and then cap all of the vials. The mid-polar fraction contains the desired alkenones, while the GDGTs are in the polar fraction. For particularly dirty or complex alkenone samples, the mid-polar fraction must be further purified with urea adduction, before analysis.
Column chromatography is widely used in chemistry as an analytical and purification technique.
Carbon nanotubes, or CNTs, are increasingly used in many industries, but there is growing concern of their effects on human health. Varying the properties of CNTs changes how they behave in water and soil. To investigate how well porous media like sand and dirt retain CNTs, a column was prepared with porous soil as the stationary phase. First, fractions were collected during loading of the CNT solution onto the column to analyze the transport of CNTs through soil. Then, the CNTs still adsorbed to the soil were eluted and the fractions analyzed for the amount of CNTs that had remained in soil. The results lend insight into the relationship between CNT surface functionalization and their transport mechanisms in the environment.
Column chromatography can be operated on both large and small scales, and is therefore used when designing syntheses for industrial applications. Spider silks have excellent tensile strength and ductility, but cannot be harvested on an industrial scale. Following silk protein synthesis, the recombinant silk proteins are purified by affinity chromatography, in which the stationary phase is designed to trap only the desired molecule. Thorough washing and elution grants the pure protein fractions needed to spin spider silk in large scales.
Many stationary phases are available for column chromatography. One stationary phase may not be suitable for all potential products of a synthesis with a broad substituent scope, such as this iodoaziridine synthesis. Crude product is mixed with various stationary phases and decomposition assessed by proton NMR. As proton NMR is highly sensitive, many stationary phases can be screened for product decomposition using a small amount of crude product. Column chromatography is then performed with the optimal stationary phase, allowing purification of novel and highly reactive compounds.
You've just watched JoVE's introduction to column chromatography for the purification of a total lipid extract. The following video will demonstrate how to further purify complex mixtures containing alkenones.
Thanks for watching!
Subscription Required. Please recommend JoVE to your librarian.
Procedure
1. Setup and Preparation of Materials
- Obtain a total lipid extract (TLE) using a solvent extraction method (Sonication, Soxhlet, or Accelerated Solvent Extraction (ASE)).
- Gather the following: combusted borosilicate glass pipettes and bulbs, silica gel, hexane, dichloromethane (DCM), and methanol.
- These materials can be purchased from any chemical retailer. The reagents should be pure and free from hydrocarbons.
- Also obtain combusted glass wool and 4-mL borosilicate glass vials.
- Make sure to have a means of supporting the column and the collection vials during the procedure. For example, a stand with clamps and a vile rack. Many labs have engineered custom-made racks that hold several columns and collection vials (Figure 2). This allows many columns to be run at the same time.
2. Methods
- Start with the dry sample in a 4-mL vial. If the sample weighs more than ~ 10 mg when dry, it may need to be split before performing the next steps as the silica gel can only react with a finite mass of organic matter.
- Suspend the sample in a small amount of hexane. This is the first and the least polar of the three solvents used in this experiment.
- If there is sample stuck to the inside of the vial, sonicate the samples for 5 min.
- Load a small amount of glass wool into the top of a pipette using a set of clean tweezers. Gently push the glass wool to the bottom of the pipette, using another pipette, to form a plug.
- Carefully transfer silica gel into the pipette until it is approximately half full.
- Place a 4-mL waste collection vial under the column.
- Soak the silica gel in the pipette with 3 volumes of hexane. This conditions the column, removes air bubbles, and rinses any impurities off the silica gel.
- Once the final wash is done, remove the waste collection vial and replace it with a vial to collect the apolar fraction.
- Carefully transfer the entire sample in hexane onto the column using a pipette. Rinse the sample vial two more times with small volumes of hexane, and transfer to the column. Allow the hexane the sample was transferred in to completely soak into the silica gel. At no time during the procedure should the silica gel dry out.
- Continue adding hexane to the top of the sample until the collection vial below the column is nearly full (~4 mL).
- Allow all of the hexane to enter the silica gel before starting with the next solvent.
- Place the mid-polarity collection vial under the column.
- Add DCM to the top of the column until the collection vial is nearly full. Again, allow all of the DCM to enter the collection vial before starting the next solvent.
- Place the polar collection vial under the column.
- Add methanol to the top of the column until the collection vile is nearly full.
Column chromatography is a flexible technique for purifying the complex mixture of compounds found in sediment. Mixtures separate as they move through the column and are collected in fractions, each containing a different chemical class of compounds. Therefore, column chromatography is often used as an additional purification step after initial isolation of the desired compound. Organic extracts such as total lipid extracts may be complex mixtures of many compounds. Some purification techniques, such as saponification, introduce compounds that can damage analytical instruments and so must be removed before analysis. This video is part of a series on lipid extraction, purification, and analysis from sediments. Once a total lipid extract is collected from a sedimentary sample, column chromatography is used to purify both alkenones and GDGTs, depending on the desired analysis.
In column chromatography, a mixture of chemical compounds is loaded onto a solid stationary phase such as silica gel. A mobile phase such as an organic solvent is then used to elute, or remove, the compounds from the column. The order in which the compounds are eluted depends on the strength of the interactions of the compounds with the silica gel and with the eluent.
The eluate is collected in fractions, each containing different compounds from the mixture. Depending on the properties of the compounds, a single solvent may provide sufficient separation and elute all of the compounds of interest. Otherwise, multiple solvents are used to elute each compound of interest in turn.
Polar compounds, which have an uneven distribution of charge, adsorb strongly to the polar silica gel, whereas apolar compounds adsorb weakly. Polar solvents have greater affinity for silica gel and therefore are more powerful eluents than apolar solvents. Thus, apolar solvents elute only apolar compounds, whereas polar solvents elute both apolar and polar compounds.
When the desired compounds are moderately polar, apolar compounds should be washed off the column with an apolar solvent before a polar solvent is used. To avoid eluting unwanted highly polar compounds such as acids, a polar eluent should not have more eluting power than needed for the most polar desired compound.
Now that you understand the principles of column chromatography, let's go through a procedure for purification of lipid biomarkers from a total lipid extract by silica gel column chromatography.
To remove organic contaminants, combust borosilicate glass pipettes, borosilicate glass vials, and glass wool for 6 h at 550 °C. Once the glassware is ready for use, set up a rack to hold pipettes and vials. Obtain pipette bulbs, clean tweezers, a laboratory spatula, silica gel, hexane, dichloromethane, and methanol. With clean tweezers, place a small tuft of glass wool into the mouth of a pipette. Gently push the glass wool to the bottom of the pipette with the stem of another pipette to form a loose plug. Carefully load silica gel into the pipette until half full. Secure the pipette upright in the rack. Secure a 4-mL borosilicate glass vial below the tip of the pipette for waste collection. In another borosilicate glass vial, suspend up to 10 mg of dry sample from the saponification process in hexane. If the sample sticks to the walls of the vial, sonicate the vial for 5 min. The chromatography procedure can now begin.
To begin the chromatography, wash the silica gel column with 3 volumes of hexane to remove air bubbles and impurities. Then, replace the waste vial with an empty vial for the apolar fraction. With a glass pipette, load the sample onto the column and allow the suspension to soak into the silica gel. Work quickly so the column does not dry out during the procedure. Rinse the sample vial twice with small portions of hexane and transfer each rinse to the column. Continue adding hexane to the column until the collection vial is nearly full. Allow all of the hexane to finish entering the silica gel. Then, exchange the filled vial with an empty vial for the mid-polar fraction. Next, rinse the sample vial with DCM and add it to the column 3 times. Continue adding DCM to the column until the collection vial is nearly full. Allow the DCM to finish soaking into the silica gel and then exchange the filled vial with an empty vial for the polar fraction. Repeat this process with methanol.
Once the vial is nearly full, allow the methanol to finish dripping into the vial and then cap all of the vials. The mid-polar fraction contains the desired alkenones, while the GDGTs are in the polar fraction. For particularly dirty or complex alkenone samples, the mid-polar fraction must be further purified with urea adduction, before analysis.
Column chromatography is widely used in chemistry as an analytical and purification technique.
Carbon nanotubes, or CNTs, are increasingly used in many industries, but there is growing concern of their effects on human health. Varying the properties of CNTs changes how they behave in water and soil. To investigate how well porous media like sand and dirt retain CNTs, a column was prepared with porous soil as the stationary phase. First, fractions were collected during loading of the CNT solution onto the column to analyze the transport of CNTs through soil. Then, the CNTs still adsorbed to the soil were eluted and the fractions analyzed for the amount of CNTs that had remained in soil. The results lend insight into the relationship between CNT surface functionalization and their transport mechanisms in the environment.
Column chromatography can be operated on both large and small scales, and is therefore used when designing syntheses for industrial applications. Spider silks have excellent tensile strength and ductility, but cannot be harvested on an industrial scale. Following silk protein synthesis, the recombinant silk proteins are purified by affinity chromatography, in which the stationary phase is designed to trap only the desired molecule. Thorough washing and elution grants the pure protein fractions needed to spin spider silk in large scales.
Many stationary phases are available for column chromatography. One stationary phase may not be suitable for all potential products of a synthesis with a broad substituent scope, such as this iodoaziridine synthesis. Crude product is mixed with various stationary phases and decomposition assessed by proton NMR. As proton NMR is highly sensitive, many stationary phases can be screened for product decomposition using a small amount of crude product. Column chromatography is then performed with the optimal stationary phase, allowing purification of novel and highly reactive compounds.
You've just watched JoVE's introduction to column chromatography for the purification of a total lipid extract. The following video will demonstrate how to further purify complex mixtures containing alkenones.
Thanks for watching!
Subscription Required. Please recommend JoVE to your librarian.
Results
This purification technique produces three different vials, each containing a different compound class or group of compound classes. The complexity of any sample to be analyzed on an instrument has been vastly decreased. This process also removes compounds, such as acids produced during a saponification, that can actually stick to parts of the instruments, because of their low volatility, which would decrease their accuracy, precision, and lifetime.
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
Alkenones and isoprenoidal GDGTs are both very common constituents of marine sediments and can be found across the world's oceans. Alkenones are being increasingly detected in lake sediments, although the organisms responsible for their production are different than in the ocean, and thus the relationship between the Uk'37 ratio and water temperature (calibration) is different from the ocean and even between separate lakes. Isoprenoidal GDGTs are found in some large lakes and just like alkenones, often need a local calibration.
The alkenones and GDGTs we are interested in come out in the ketone and polar fractions, respectively. In marine sediments we often analyze both sea surface temperature (SST) proxies from one sample. This allows the construction of two independent SST records, which show the evolution of water temperature at the core site through time. This comparison, called a multi-proxy approach, often highlights times when the two proxies agree and times when they don't. This agreement or discrepancy itself contains information. If the two proxies agree, maybe the producing organisms occupied the same depth habitat, or maybe they lived at separate depths but a well-mixed water column led to the vertical homogenization of temperature (water usually cools with depth). If the two proxies disagree, it could be that the two populations lived at separate depths; one living in warm, shallow waters and one in cooler, deeper water. Or it could be that the compounds were produced during different times of the year and so reflect the temperatures of different seasons. These questions are created by the analysis of two different SST proxies at the same site and they highlight the care organic geochemists and paleo-climatologists need to take when interpreting their data.
Because of the high relative stability of apolar hydrocarbons, the apolar fraction contains many interesting organic compounds. Alkanes are important constituents of a leaf's outer waxy layer and they are used in sediment records for many reasons. Their average chain length (number of carbon atoms) contains information on the dominance of aquatic vs. terrestrial plants, temperature, and precipitation. The isotopic ratio of carbon in alkanes is related to the C3 vs. C4 plant-type of the plant that produced it and the hydrogen isotopic ratio is related to local to global temperature and precipitation. Steranes and hopanes are also found in the apolar fraction. These biomarkers are the geostable versions of bioactive compounds like hopanoids and steroids, which serve important biochemical roles in prokaryotes and eukaryotes, respectively.
The mid-polarity fraction contains our alkenones. Alkenones are ketones, which are important recorders of ancient surface temperatures via the Uk'37 sea surface temperature proxy. Some ketones also come from the same leaf waxes the alkanes do, although there are generally far less.
The polar fraction contains carboxylic acids, another important constituent in leaf wax, that is slightly less specific and harder to work with than alkanes (low volatility) but can nonetheless relate some of the same information. Glycerol dialkyl glycerol tetraethers (GDGTs) are in the polar fraction and are another important recorder of ancient water and air temperatures.
Subscription Required. Please recommend JoVE to your librarian.
References
Killops, S. and Killops, V. Introduction to Organic Geochemistry. Blackwell Publishing, Malden, MA (2005).




