Overview
Robert M Rioux, Pennsylvania State University, University Park, PA
The use of gases in a synthetic chemistry laboratory is essential for carrying out a variety of highly facile and atom economical transformations. Reactions such as hydrogenation, oxidation, and amination require the use of gases like hydrogen, oxygen, and ammonia. Due to the poor solubility of these gases in typical reactant solutions, high pressures are necessary to achieve a meaningful reaction rate. Not only are these gases highly reactive, the use of high pressures makes these operations fairly hazardous. The biggest challenge in the use of high pressure is the containment of the high-pressure gas for the entire duration of the reaction, with close monitoring of the pressure and temperature, to avoid the formation of explosive mixtures and runaway reactions.
These reactions are typically carried out using thick-walled pressure vessels. The pressurized vessel allows for operation at high pressure with appropriate safety concerns abated. Figure 1 demonstrates the various parts of a typical pressure vessel, used to conduct high-pressure reactions. The following protocol highlights the procedure for the safe operation of these high-pressure reactor vessels.
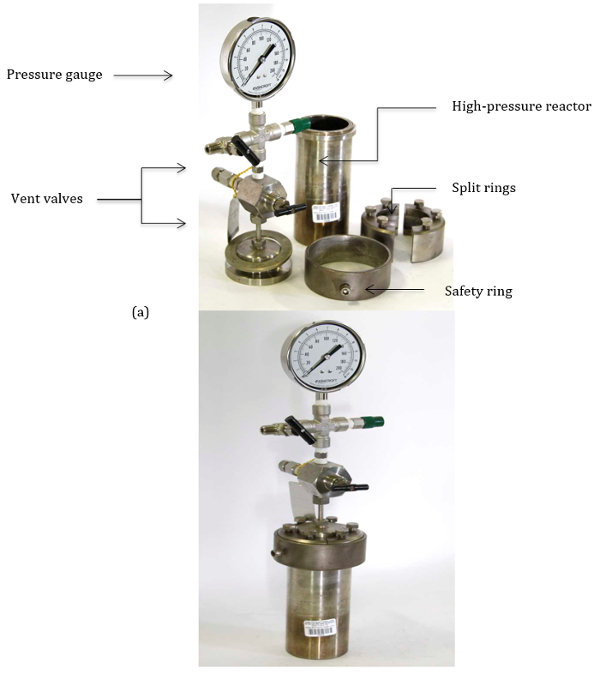
Figure 1. (a) Parts of the high pressure reactor vessel. (b) Assembled high pressure reactor vessel.
Procedure
The operation of the high-pressure Parr reactor (or equivalent) can be roughly broken down into 3 steps.
1. Charging
- Select an appropriate secondary reaction vessel based on the scale of the reaction. Test tubes, Erlenmeyer flasks, or round-bottom flasks are some examples of appropriate reaction vessels. Make sure to keep enough head space above the solvent level in the flask as the solvent tends to bubble up during pressure venting.
- Place the reactants, along with a stir bar, in the reaction vessel and place it in the high-pressure reactor.
- Place the pressure gauge assembly on the top of the reaction vessel. Make sure the vent valve is completely closed. Turn it clockwise to finger tight. Do not overtighten.
- Assemble the split rings on the vessel.
- Start tightening the diagonally opposite screws on the split rings, but do not tighten them all the way. This is to ensure that the pressure exerted by the gauge is even across the vessel.
- Tighten all screws completely.
- Place the safety ring on the bench, and place the reactor in the ring.
- Slide the ring up to the split rings, and align the screw with the dent on the side of the split ring.
- Finger tighten the safety ring.
- The vessel is now ready for the next stage.
2. Purging and Pressurizing
- Attach the pressurized gas source to the reactor and turn on the main valve on the regulator.
- Set the pressure to approximately 1/3rd of the final required pressure.
- Slowly open the vent valve on the pressure gauge and pressurize the reactor.
- Now close the main valve on the gas regulator.
- Slowly loosen the pressure line going in to the reactor, so that the pressure in the reactor starts to fall. Make sure the reactor is in a well-ventilated area.
- Once the pressure falls back to zero, tighten the pressure line again, and open the main valve on the gas regulator.
- Adjust the pressure to 2/3 of the final required pressure and repeat steps 3-6 above.
- Now adjust the pressure on the regulator to the final desired value and pressurize the reactor.
- Once the final pressure is reached, close the vent valve on the pressure gauge, and close the main valve on the gas regulator.
- Carefully loosen the pressure line, so that the gas in the line and the regulator is vented.
- Always set the outlet pressure on the gas regulator back to zero (this usually means loosening the pressure control valve). This ensures that gas will not leak, even if the main valve on the regulator is turned on by accident.
- Now place the reactor in a hood and let the reaction run for the desired amount of time. The reactor can be heated if desired. Ensure the temperature is below the rated limit of the vessel.
3. Venting
- Once the reaction time is over, cool the reactor to room temperature, if necessary.
- Now slowly open the vent valve on the gauge to vent the gas from the reactor. Do this as slowly as possible to prevent the solvent spilling over in the reactor. Crucially, make sure the reactor is in a fume hood.
- Once the pressure in the reactor drops to zero, loosen the safety ring and the screws on the split rings.
- Disassemble the split rings and remove the gauge from the reactor.
- Remove the reaction vessel from the reactor.
- Once the reaction vessel is removed from the reactor, rinse the reactor with water and then acetone and leave it open to dry.
The use of gases in the synthetic chemistry laboratory is essential for carrying out a variety of highly facile and atom economical transformations, and often require high pressures to ensure sufficient solubility of gases into the reactant solution.
Reactions such as hydrogenation, oxidation, and amination require the use of gases like hydrogen, oxygen, and ammonia, respectively. Due to the poor solubility of these gases in typical reactant solutions, high pressures are necessary to achieve a meaningful reaction rate. Therefore, high-pressure reactor vessels - thick-walled containers, typically made of stainless steel - are used to carry out such reactions. The pressurized vessel allows for operation at high pressure with appropriate safety concerns abated.
In this video, we will first review the safety considerations and then learn how to charge, purge, and vent a high-pressure reactor vessel.
High-pressure reactor vessels can maintain environments of 3,000 PSI and 500 degrees. Vessels rated for higher pressures require thicker walls, though, making temperature control more difficult.
The manufacturer's limits must be maintained during operation, as the gases are highly reactive, as well as the high pressure being a hazard itself. In addition to temperature and pressure, capacity and corrosion resistance must also be kept in mind when setting up an experiment.
The reaction itself must also be considered, as some reactions, like Hydroformylation, produce heat or while others like the Haber-Bosch-Process result in gaseous products. Too much heat or gas formation could push the reactor outside its operating limits leading to an explosion.
With these safety considerations in mind, let's see how to work with these vessels.
To begin the procedure, select a clean secondary vessel in which the reaction will take place. Depending on the reaction's scale, this can be a test tube, Erlenmeyer, or round-bottomed flask.
Add the reactants along with a clean stirbar into the secondary vessel.
Place the pressure gauge assembly on top of the reaction vessel. Close the vent valve by turning it clockwise until finger tight.
Add the split rings onto the vessel, tightening diagonally opposite screws to seal the reactor. Do not tighten the screws all at once to ensure even pressure across the vessel.
Place the reactor inside the safety ring on the benchtop. Slide the ring over the split rings, and align the screw with the indent on the split ring.
Finger tighten the safety ring. With the reactor sealed, it is ready to be purged and pressurized.
The next step is to purge the affixed reactor with an inert gas. Attach the gas source to the reactor and open the main valve on the regulator.
Using the cylinder regulator set the pressure to approximately 1/3rd of the final required value. Slowly open the vent valve on the pressure gauge and pressurize the reactor.
When desired pressure is reached, close the valve to the autoclave, followed by the valve to the gas source on the regulator and the cylinder valve.
Slowly loosen the pressure line going into the reactor, so that the pressure in the reactor starts to fall. Once the pressure falls back to zero, close the pressure line again and open the main valve on the regulator to the gas source.
Repeat the previous process with 2/3rd of the final pressure.
Now adjust the pressure on the regulator to the final desired value and pressurize the reactor. Once the final pressure is reached, close the vent valve on the pressure gauge, and close the main valve on the gas regulator.
Carefully loosen the pressure line, so that the gas in the line and the regulator is vented. This ensures that the gas source is disconnected from the reactor, which is important, once chemistry has been initiated.
Set the outlet pressure on the cylinder regulator back to zero by loosening the pressure control valve. This ensures that gas will not leak, even if the main valve on the regulator is turned on by accident.
Now place the reactor in a fume hood and let the reaction run for the desired amount of time. The reactor can be heated if desired.
The next step is to safely vent the completed reaction. Once the reaction time has elapsed, cool the reactor to room temperature.
Then, slowly open the vent valve on the gauge to vent the gas from the reactor. Do this as slow as possible to avoid the solvent from spilling over in the reactor.
Once the pressure in the reactor has dropped to zero, loosen the safety ring and the screws on the split rings. Disassemble the split rings and remove the gauge from the reactor.
Collect the reaction vessel from the reactor. Rinse the reactor with water and the acetone. Leave it open to air dry.
You've just watched JoVE's introduction to using high-pressure reactor vessels. You should now understand their function, and how to properly charge, pressurize, and vent one. Thanks for watching!
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
The manipulation of gases at high pressure can be done with the use of a Parr reactor (or equivalent) vessel. Proper safety precautions should be observed while operating these vessels as they present an explosion hazard.
Subscription Required. Please recommend JoVE to your librarian.




