Summary
Here, we present a protocol to study the immunology of rejection. The surgical model presented reports a short operating time and a concise technique. Depending on the donor-recipient strain combination, the transplanted kidney may develop acute cellular rejection or chronic allograft damage, defined by interstitial fibrosis and tubular atrophy.
Abstract
Rejection of the transplanted kidney in humans is still a major cause of morbidity and mortality. The mouse model of renal transplantation closely replicates both the technical and pathological processes that occur in human renal transplantation. Although mouse models of allogeneic rejection in organs other than the kidney exist, and are more technically feasible, there is evidence that different organs elicit disparate rejection modes and dynamics, for instance the time course of rejection in cardiac and renal allograft differs significantly in certain strain combinations. This model is an attractive tool for many reasons despite its technical challenges. As inbred mouse strain haplotypes are well characterized it is possible to choose donor and recipient combinations to model acute allograft rejection by transplanting across MHC class I and II loci. Conversely by transplanting between strains with similar haplotypes a chronic process can be elicited were the allograft kidney develops interstitial fibrosis and tubular atrophy. We have modified the surgical technique to reduce operating time and improve ease of surgery, however a learning curve still needs to be overcome in order to faithfully replicate the model. This study will provide key points in the surgical procedure and aid the process of establishing this technique.
Introduction
Successful renal transplantation for the treatment of renal failure was first described in 1955 between monozygotic twins1, since then it has become a revolutionary treatment for patients with end-stage renal failure throughout the world, offering both improvement in length and quality of life2. However long term graft survival has been hampered by a multitude of pathological processes resulting in chronic allograft damage3.
Rejection of the transplanted kidney in humans remains a major cause of morbidity, despite significant improvements in immunosupporessive regimens. The aim of developing a mouse model of renal transplantation is to closely replicate the process and pathology found in human renal transplantation4. Skoskiewicz et al. first described the mouse model of renal transplantation in 19735. Although advanced microsurgical skills are required, it is a valuable tool for several reasons: the mouse genome has been well characterized and there is a great variety of experimental methods and techniques available for mouse studies.
Many groups using the mouse model of renal transplantation have used the transplanted kidney as a life-supporting organ, however in other studies and in our described methodology one of the recipient mouse’s native kidneys is left in situ for the duration of the experiment4. The benefit is that the mouse undergoes a single anesthesia and operation thereby reducing the morbidity to the mouse and the risk of death from a second procedure. Additionally the mouse does not suffer from the adverse effects of gradual renal failure.
Although models of allogeneic rejection exist in other organs such as the heart and skin, these are not always directly relevant to renal transplantation. There is evidence that these models elicit different modes and dynamics of rejection, for instance the time course of rejection in cardiac allograft and renal allograft differs significantly in certain strain combinations6. We have described acute renal allograft rejection patterns in BALB/c donors into non-transgenic FVB/nj mice, this model showed cellular mediated injury with accumulation of T cells and macrophages7. Alternatively we have also described a model of chronic allograft damage that exhibits interstitial fibrosis and tubular atrophy, this results from transplanting a kidney from C57BL/6BM12 donors into C57BL/6 recipients, as these mice are characterized by a single MHC class II loci mis-match8.
Multiple aspects of transplantation have been studied using the mouse model of renal transplantation including acute rejection, cellular and humoral rejection, ischemia reperfusion injury, and trialing novel therapeutic agents. We have modified the surgical technique to reduce operating time and improve the ease of surgery. Particularly we have described simultaneous donor and recipient preparation and a simplified vascular anastomosis technique by utilizing a continuous aortic patch anastomosis. This video and manuscript will provide key points to aid in the establishment of this technique.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
Appropriate national and local institutional ethics should be in place before performing animal experiments. Specifically in the United Kingdom the following experiments were undertaken under the Animals (Scientific Procedures) Act 1986. Where two microsurgeons are available to operate simultaneously the donor surgeon should perform steps 1.1 to 1.16 then 3.1 to 3.5, whilst the recipient surgeon performs 2.1 to 2.8. For a single operator the steps can be followed sequentially.
1. Donor Preparation
Note: The procedure presented here is for a donor C57BL/6BM12 and recipient C57BL/6 male mice aged between 8 to 16 weeks old with a body weight greater than 20 g. However this procedure can be reproducibly performed on a variety of mouse strains. The data presented in the representative results section was obtained from C57BL/6, C57BL/6BM12 and BALB/c mice.
- Conduct procedures using sterile surgical instruments and consumables (autoclaved), with endeavors to keep the operating area as sterile as possible. Perform the donor preparation simultaneously with the recipient preparation if two surgeons are available.
- Anesthetize the mouse with an intraperitoneal injection (31G needle) of medetomidine (0.5 mg/kg) and ketamine hydrochloride (200 mg/kg). This results in an anesthetic plane that is maintained for 4 hr providing enough time for the whole procedure to be performed. Supplemental anesthesia is not necessary.
- Confirm that the mouse is anaesthetized (no response to toe pinch).
- Shave the mouse’s abdomen and remove any loose hair with sticky tape.
- Place the mouse on its back on a sterilely draped heated mat and loosely immobilize the limbs with sterile masking tape.
- Monitor the mouse throughout the procedure for thermal burns. If possible use a non-electric heat source.
- Apply an eye lubricant and sanitize the abdominal wall with a dilute iodine solution.
- Make a midline incision in the abdomen to enter the peritoneal cavity and insert a 3 cm Calibri abdominal retractor.
- Apply warmed saline to keep the intestines and surgical area moist and avoid unnecessary drying of the viscera.
- Cover the mouse with sterile drapes and move the intestines to the operator’s left (mouse’s right) to expose the aorta, vena cava and left kidney.
- Apply a ratcheted forcep to the stomach and pull superiorly to expose the major vessels and left kidney fully. Pack damp sterile swabs (2 mm x 2 mm) into the abdomen to retract tissues away from the surgical area, such as the liver lobes, seminal vesicles, and bowel.
- Isolate the left kidney from surrounding adventia, fat and the left adrenal gland in the peritoneal cavity by bluntly dissecting the connective tissue using fine tip forceps. Place the closed tips of the forceps between the areas that need to be separated and slowly allow the forcep tips to open to dissect the space.
- Isolate the left renal vein by ligating and then dividing the left adrenal vein and left gonadal vein with 9/O nylon. Place the suture close to the renal vein.
- Ligate and divide the ureter with 7/O silk suture close to the bladder leaving the suture ends long. These long suture ends will be used when the kidney is harvested and are necessary for the ureteric anastomosis.
- To mobilize and fully dissect the aorta and vena cava superiorly and inferior to the renal artery and vein use fine tip forceps to bluntly dissect lymphatic vessels and fat from the front and sides of the vessels.
- Find the tissue plane between the aorta and vena cava and slowly spread the forceps. Slowly open the tips of the fine tipped forceps to spread the tissue with minimal trauma.
- Tie a loose 7/O silk around the aorta superior and inferior to the renal artery by passing an angled fine tip forceps around the back of the vessels and drawing a suture through. These sutures will be tightened prior to kidney retrieval to allow retrograde perfusion of University of Wisconsin solution.
2. Recipient Preparation
As per the donor preparation follow steps 1.2 to 1.8.
- Move the intestines to the operator’s right (mouse’s left) to expose the aorta, vena cava. Cover the intestines with a sterile saline soaked swab.
- Perform a right nephrectomy by ligating the right renal artery and vein together with 7/0 silk suture and then divide.
- Ligate the right ureter with 7/O silk suture and divide.
- Mobilize and fully dissect the aorta and vena cava inferior to the renal artery and vein as described in step 1.14 and 1.15. Ensure complete dissection between the aorta and vena cava. Take care to preserve the internal spermatic artery which runs anterior to the vena cava and aorta along with the lymphatic bundle.
- Identify lumbar vessels running in the retroperitoneum from the vena cava and vein to the spine, ligate with 9/O silk in continuity, there is no need to divide the vessels.
- Identify sufficient space to place microvascular clamps with space between for vascular anastomoses.
- Administer intravenous heparin (5 units) via dorsal penile vein.
3. Donor Kidney Retrieval
Tighten the 7/O silk ties that have been placed around the inferior and superior aorta to isolate the kidney from the arterial circulation.
- Infuse retrograde cold 0.2 – 0.5 ml of University of Wisconsin solution with a needle (31G) into the aorta.
- Divide the aorta within the sutures and divide the renal vein at its junction with the vena cava to remove the kidney and the renal artery with a length of aorta. Divide lumbar vessels arising from the aorta, if they are present, without ligation.
- Place the kidney in cold saline on a sterile swab in a culture dish.
- Euthanize the donor mouse by cervical dislocation.
4. Kidney Transplantation – Kidney Preparation
- Create an aortic patch by dividing the aorta wall longitudinally directly opposite the renal artery. Identify any vessel lumens in the patch that need to be ligated or avoided when performing the arterial anastomosis.
- Place a 10/O suture from outside to inside the renal vein lumen superiorly and a second separate suture inferiorly. The sutures used to divide the adrenal vein and gonadal vein can be used to orientate the vessel.
- Place the kidney in the right flank of the recipient (Figure 1.1) and ensure the sutures are well placed to ensure vascular anastomoses can be completed without the sutures becoming entwined in swabs or other instruments.
5. Kidney Transplantation – Vascular Anastomosis
Apply microvascular clamps first inferiorly then superiorly encompassing the vena cava and aorta.
- Make a ventomy with a needle (31G) by puncturing the anterior wall of the vena cava. Flush blood from the vena cava by injecting approximately 50 μl of 0.9% NaCl.
- Widen the venotomy using fine tip forceps by opening them within the venotomy so that the length is equivalent to the diameter of the donor renal vein.
- Place the 10/O superior suture (which is already in the lumen of the renal vein) first at the apex of the venotomy (Figure 1.2) and join the back wall of the anastomosis in a running suture until the inferior apex is reached (Figure 1.3).
- Place the 10/O inferior suture and tie first with a single knot (Figure 1.4) then tie to the running suture from the back wall.
- Using the inferior suture create the front wall of the anastomosis (Figure 1.5) with a running suture and tie to the superior suture end at the superior apex (Figure 1.6).
- Create an aortotomy by picking up the aorta wall with forceps and cutting an elliptical patch with scissors (approximately the aortotomy should be a fifth of the circumference of the aorta and three times the renal artery lumen in length).
- Place a 10/O suture at the superior point of the aortic patch from outside to inside (Figure 1.7) and pass through the aorta to tie outside the vessels at the superior apex of the aortotomy.
- Create the arterial anastomosis with a running suture starting superiorly (Figure 1.8) anastomosing the donor patch to the recipient aorta, take care not to constrict the anastomosis by tying the suture too tight (Figure 1.9).
- Remove the inferior vascular clamps first then the superior clamp to reperfuse the kidney (Figure 1.10 and 1.11). Visible peristalsis of the ureter maybe seen if adequate blood supply to the ureter is achieved.
6. Kidney Transplantation – Ureteric Anastomosis
- Divide any attachments of the bladder from the abdominal wall.
- Pass a needle (21G) from the left to right of the bladder through both walls.
- Place straight forcep tips in the needle lumen and pass both back through the bladder such that the forceps are now passing from right to left out of the left side of the bladder.
- Draw the suture on the donor ureter through the bladder such that the ureter passes in the left bladder defect then out of the right side.
- Suture the adventitia of the ureter to the adventitia of the bladder with three single interrupted sutures around the entry point with 9/O nylon suture on a round-bodied needle.
- Cut the ureter proximal to the ligature, thus opening the ureter to allow urine to flow and allow the ureter end to retract into the body of the bladder. Visible production of urine may be observed along with bleeding from peri-ureteric vessels.
- Close the right side bladder defect with a single interrupted 9/O silk suture.
7. Recovery and Post-operative Care
Replace the gastrointestinal viscera into the abdomen in their original orientation and close the abdominal wall by approximating the rectus muscles with 6/O absorbable suture.
- Approximate the skin with metal skin clips.
- Partially reverse the anesthesia with a subcutaneous injection of atipamazole hydrochloride (10 μl/g).
- Administer analgesia by subcutaneous buprenorphine hydrochloride (0.05 mg/kg) and for fluid support injection 1 ml of subcutaneous 0.9% NaCl.
- Recover the mouse in a warming cabinet at 28 °C for 24 to 48 hr. Observe the mouse for illness up to the completion of the experiment.
- Remove metal skin clips 7 – 10 days post operatively.
- Once the experiment is complete euthanize the mouse by cervical dislocation.
- Remove and collect the contralateral kidney and allograft for histological analysis.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Renal allograft rejection can be assessed by histological analysis of methacarn-fixed paraffin-embedded tissue sections of the transplanted kidney (Figure 2). Isograft transplantation of kidneys between syngeneic mice results in renal ischemic reperfusion injury, however by 4 weeks the tubules have recovered and are histologically comparable to native kidneys. Acute rejection can be modeled by C57BL/6 kidney transplantation into BALB/c recipients, within 1 week there is diffuse mononuclear cell infiltration throughout the renal parenchyma, involving the interstitium, glomeruli and tubules. Chronic allograft damage can be modeled by C57BL/6BM12 kidneys transplanted into C57BL/6 recipients, this results in the typical features found in human pathology consisting of interstitial fibrosis and gradual tubule loss. Gross tubule count per high-powered field (x200 magnification) allows quantification of the functional nephron mass (Figure 3), loss of tubules reflects tubular injury due to rejection. Interstitial fibrosis can be identified using the pan-collagen stain picrosirius red (Figure 4). Normal endogenous collagen is observed, however during chronic damage new collagen is deposited resulting in progressive fibrosis. The chronic damage becomes apparent between 8 and 12 weeks after transplantation (Figure 5).
A significant learning curve needs to be overcome in order to establish the model (Figure 6). An estimated 40 procedures were performed in recovered mice before a reproducible vascular anastomosis time was reached with acceptable survival free of complications. The most common reason for euthanizing the mouse was due to hind limb paralysis secondary to lower-limb and spinal cord ischemia related to arterial thrombosis, however in our experience systemic heparization reduces the incidence of this. Mice were routinely monitored according to locally agreed criteria for terminating the experiments. Survival experiments performed in the plateau phase of the learning curve resulted in a mean vascular anastomosis time of 28.9 ± 0.47 min.
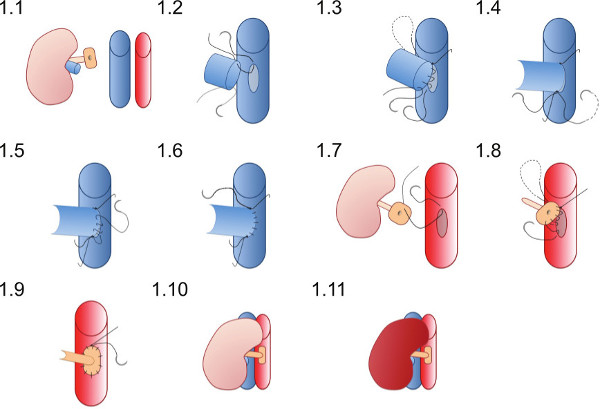
Figure 1. Diagrammatic representation of vascular anastomosis technique. The donor kidney is placed in the right flank of the recipient mouse. The donor renal vein is anastomosed in an end-to-side fashion and the donor renal artery on a patch of aorta is anastomosed to the recipient aorta.

Figure 2. Representative histological tubular injury in the transplanted kidney. After fixation in methyl Carnoyl’s solution tissues were embedded in paraffin and tissue sections of 4μm were stained by Hemotoxylin and Eosin. At 4 weeks following transplantation isograft kidneys do not exhibit tubular injury and are comparable to native kidneys in appearance. C57BL/6BM12 kidneys transplanted into BALB/c recipients undergo acute allograft rejection with diffuse mononuclear cell infiltrates (*) and necrotic tubules (**) and tubulitis. C57BL/6BM12 kidneys transplanted into C57BL/6 results in chronic allograft damage characterized by perivascular lymphocytic infiltrates (block arrow ⬆) and interstitial fibrosis and tubular atrophy (hollow arrows ⇧). Please click here to view a larger version of this figure.

Figure 3. Quantification of tubular injury in the transplanted kidney. Healthy tubules (defined by the presence of an intact basement membrane, intact tubule lumen, healthy cytoplasmic volume and a maintained apical microvilli brush border) reflecting functioning nephron mass can be quantified by counting the average number of tubules per field (x200 magnification) (n = 6, average of 10 consecutive fields, ** p < 0.01).

Figure 4. Chronic allograft damage can be identified by interstitial fibrosis (representative images). Collagen deposition within the transplanted kidney is apparent by detection of picrosirius red staining of collagen. Please click here to view a larger version of this figure.

Figure 5. Quantification of interstitial fibrosis. Interstitial fibrosis measured by picrosirius red positivity is raised in C57BL/6BM12 ⇒ C57BL/6 recipients twelve weeks following transplantation (n = 6, average of 10 consecutive fields, ** p < 0.01, *p < 0.05).

Figure 6. Learning curve. Initial non-recovery experiments in terminally anaesthetized mice were performed to develop the surgical technique. This was followed by recovery experiments to achieve a successfully re-perfused transplanted kidney with a surviving mouse without complications.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
The most well described manner to perform the arterial anastomosis is to use the distal aorta of the donor, with the renal artery in continuation, in an end-to-side manner to the recipient aorta. We describe the use of an aortic patch, similar to the ‘Carrell patch’ mirroring that performed in human kidney transplantation which we believe to be more convenient. Though reports in the literature of donor and recipient operative time are sparse we believe that utilizing an aortic patch to recipient aorta instead of end-to-side donor aorta to recipient aorta is preferable. Using a patch negates the need to dissect all lumbar donor aortic branches, and also the time taken to ligate them individually, as anastomosing a patch naturally excludes these.
Critical steps to achieve this model include taking care to ensure that there are no other arterial lumens other than the renal artery within the arterial patch anastomosis as they will leak when micro-vascular clamps are removed. The sutures constructing the anastamoses can be tied tightly but be aware of the purse-string effect which can narrow the anastomosis and cause ischemia or inhibit any blood flow, in practicality we have observed that provided the elliptical aortotomy is of sufficient size the purse-string effect is not encountered. The venotomy is made with a needle then widened by stretching with forceps, we believe this is preferable to cutting as stretching with forceps creates a ring of venous tissue which can be easily seen and aid suturing to create a stable anastomosis. The ureteric anastomosis to the bladder is a very important component to consider, we advocate the technique described here rather than a bladder-dome to bladder re-construction that has been associated with urine leak and ureteric stenosis presumably due ischemia. Our described technique allows a shorter donor ureteric length to be used as it is directly anastomosed to the bladder5. By utilizing these technical improvements we have been able to significantly reduce the amount of time required to complete this experimental model. Indeed with two operators working simultaneously five transplant recipients can be performed per day with sufficient time to allow recovery and monitoring of the mice.
A major limitation of the described model and technique in this paper is that the mouse is left with one of its native kidneys in situ such that the mouse is not dependent on the kidney for survival. Some authors have reported the removal of the second native kidney immediately at the time of transplant or five to ten days after transplantation, thus leaving the mouse reliant on the transplanted kidney. This would allow survival to be used as an experimental outcome as well as sampling blood to measure markers of renal function such as serum creatinine or blood urea. However there is no difference in histological outcomes when comparing mice dependent on the allograft and those with a single native kidney9. Our described protocol does not preclude this and indeed the second native kidney could be removed at a given time interval. An additional limitation is that advanced micro-surgical expertise is required in order to perform an arterial and venous anastomosis, though with training and by utilizing the technical figure for instruction this can be overcome. Vascular complications can occur, these being renal artery or vein thrombosis, this invariably results in the mouse displaying signs of distress, ill-health or specifically hind-limb paralysis. Therefore strict governance and monitoring of mice is imperative when utilizing this model.
Acute rejection of the renal transplant has largely been treated by immunosuppression and induction therapy depleting circulating lymphocytes, however episodes of cell-mediated rejection can still occur throughout the renal grafts life. Therefore studies of the mechanisms underlying this are still pertinent and may identify novel pathways for treatment. In complete MHC mismatch, such as C57BL/6 into BALB/c, mean survival dependent on the kidney transplant has been reported to be as low as 7.4 days10. Histologically acute cellular and vascular rejection can be identified by lymphocytic infiltration, hemorrhage and edema in the interstitium, tubulitis, vasculitis, with glomerular and tubular necrosis. The combined processes contributing to chronic allograft damage have made this area difficult to study. The histological features include interstitial fibrosis, tubular atrophy, glomerulosclerosis and intimal proliferation. The progressive injury is associated with persistent T cell infiltration, however it is increasingly appreciated that many other factors may be involved. Potential mediates of persistent injury include complement deposition due to donor-specific antibodies, B cells, natural killer cells, macrophages, and cells intrinsic to the graft such as the endothelium3. Therefore this model allows the study of these various facets of rejection.
There have been approximately 70 published studies utilizing this model despite its early description4, this is in comparison to mouse models of kidney ischemia-reperfusion injury where there have been several hundred articles. The importance of this mouse model of intra-abdominal kidney transplantation is that it directly recreates the process of human kidney transplantation, furthermore it benefits from the use of well-defined inbred mouse strains that can be used to model different mechanisms of rejection. Hence the studies using this model are highly translatable. Other future applications of this model include studying intrinsic renal abnormalities by transplanting kidneys with specific phenotypes from knockout models into wild-type mice or conversely wild-type kidneys in genetically altered recipients.
This model can successfully replicate the process of human renal transplantation. The use of inbred mouse strains allows selection of donor-recipient combinations of varying MHC differences. Furthermore the use of mice allows the use of various techniques including knockout and inducible systems to probe the different aspects of rejection.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Funding from Kidney Research UK, The Royal College of Surgeons of Edinburgh and The European Society of Organ Transplantation supported this study.
Materials
| Name | Company | Catalog Number | Comments |
| Surgical Instruments | |||
| Blunt Dissecting Scissors | Fine Science Tools | 14072-10 | For skin cutting |
| Curved Castoviejo scissors | Fine Science Tools | 15017-10 | For tissue cutting |
| Spring Scissors – straight | Fine Science Tools | 15000-08 | For suture cutting |
| Toothed forceps 1x2 teeth | Fine Science Tools | 11021-12 | |
| 2 x Fine Tip forceps (Dumont No.5) | Fine Science Tools | 11251-20 | |
| Angled Fine Tip forceps (Dumont No. 5/45) | Fine Science Tools | 11253-25 | For blunt dissecting |
| Curved Fine Tip forcep (Dumont No.7) | Fine Science Tools | 11273-22 | Useful to pass around vessels |
| Curved Crile Haemostat | Fine Science Tools | 1300-04 | |
| Micro clip applicator with lock | Fine Science Tools | 18056-14 | |
| 2 x Micro serrefines spring width 2mm, jaw length 4mm | Fine Science Tools | 18055-04 | Microvascular clamps |
| 2 x Colibri 3cm wire retractor | Fine Science Tools | 17000-03 | |
| Castroviejo needle holder with lock | Fine Science Tools | 120660-01 | |
| Wound clip applicator | Fine Science Tools | 12031-07 | |
| 7mm wound clips | Fine Science Tools | 12032-07 | Remove 7 to 10 days after surgery |
| Equipment | |||
| OPMI pico microscope | Carl Zeiss | S100 | |
| Thermal cautery unit with fine tip | Geiger | 150A | |
| Heat electronic pad | Cozee Cumfort | n/a | |
| Euroklav 23-S | Melag | n/a | Autoclave |
| Disposable equipment | |||
| 7/O Silk braided suture | Pearsall | 30514 | |
| 10/O Dafilon (polyamide) suture | B-Braun | G1118099 | |
| 6/O Vicryl (plygalectin) | Ethicon | W9537 | |
| Regular bevel needle, 1 inch, 21G | Bection, Dickinson and Company | 305175 | For ureteric anastamosis |
| Regular bevel needle, 5/8 inch, 25G | Bection, Dickinson and Company | 305122 | |
| Regular bevel needle, 1/2 inch, 30G | Bection, Dickinson and Company | 304000 | |
| Insulin needle 1ml, 29G | Bection, Dickinson and Company | 324827 | |
| Insulin needle 0.3ml, 30G | Bection, Dickinson and Company | 324826 | |
| 1 ml syringe slip tip | Bection, Dickinson and Company | 300184 | |
| 5 ml syringe slip tip | Bection, Dickinson and Company | 302187 | |
| Wypall paper swabs | Kimberley-Clark | L40 | sterilised by autoclave |
| Cotton wool buds | Johnson and Johnson | n/a | sterilised by autoclave |
| Plain drapes | Guardian | CB03 | sterilised by autoclave |
| Cell culture dish 60mm x 15mm | Corning Incorporated | 430166 | |
| Dispensing Pin | B-Braun | DP3500L / 413501 | Used with NaCl 0.9% |
| Re-agents and Drugs | |||
| (Lacri-Lube) White soft paraffin 57.3%, mineral oil 42.5% and lanolin alcohols 0.2% | Allergan Ltd | 21956GB10X | |
| (Videne) Povidone-iodine 10% | Ecolab Ltd | PL 04509/0041 | |
| (Vetalar V) Ketamine hydrochloride | Pfizer Animal Health | Vm 42058/4165 | 100mg/ml solution (dose 200mg/kg) |
| (Domitor) Medetomidine hydrochloride | Orion Pharma | Vm 06043/4003 | 1mg/ml (dose 0.5mg/kg) |
| (Vetergesic) Bupernorphine hydrochloride | Alsto Animal Health | Vm 00063/4002 | 0.3mg/ml (dose 0.05mg/kg) |
| (Antisedan) Atipamezole hydrochoride | Orion Pharma | Vm 06043/4004 | 5mg/ml (dose 2mg/kg) |
| University of Wisconsin Solution | Belzer Bridge to Life | n/a | dose approximately 500 microlitres/mouse |
| NaCl 0.9% | Baxter | FKE1323 | |
| Heparin Sulphate | non-proprietary | n/a | 5000units/ml (dose 5units/mouse) |
References
- Guild, W. R., Harrison, J. H., Merrill, J. P., Murray, J. Successful homotransplantation of the kidney in an identical twin. Trans. Am. Clin. Climatol Assoc. 67, 167-173 (1955).
- Wolfe, R. A., et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725-1730 (1999).
- Nankivell, B. J., Alexander, S. I. Rejection of the Kidney Allograft. N. Engl. J. Med. 363, 1451-1462 (2010).
- Tse, G. H., Hughes, J., Marson, L. P. Systematic review of mouse kidney transplantation. Transplant International. 26, 1149-1160 (2013).
- Skoskiewicz, M., Chase, C., Winn, H. J., Russell, P. S. Kidney transplants between mice of graded immunogenetic diversity. Transplant. Proc. 5, 721-725 (1973).
- Zhang, Z., et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 62, 1267-1272 (1996).
- Qi, F., et al. Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation. 86, 1267-1274 (2008).
- Dang, Z., Mackinnon, A., Marson, L. P., Sethi, T. Tubular atrophy and interstitial fibrosis after renal transplantation is dependent on galectin-3. Transplantation. 93, 477-484 (2012).
- Jabs, W. J., et al. Heterogeneity in the Evolution and Mechanisms of the Lesions of Kidney Allograft Rejection in Mice. Am. J. Transplant. 3, 1501-1509 (2003).
- Lin, T., et al. Deficiency of C4 from Donor or Recipient Mouse Fails to Prevent Renal Allograft Rejection. Am. J. Pathol. 168, 1241-1248 (2006).



