Summary
An original experimental setup for heating cells in a culture dish using 1.94 µm continuous-wave laser radiation is introduced here. Using this method, the biological responses of retinal pigment epithelial (RPE) cells after different thermal exposures can be investigated.
Abstract
An original method to heat cultured cells using a 1.94 µm continuous-wave thulium laser for biological assessment is introduced here. Thulium laser radiation is strongly absorbed by water, and the cells at the bottom of the culture dish are heated through thermal diffusion. A laser fiber with a diameter of 365 µm is set about 12 cm above the culture dish, without any optics, such that the laser beam diameter is almost equivalent to the inner diameter of the culture dish (30 mm). By keeping a consistent amount of culture medium in each experiment, it is possible to irradiate the cells with a highly reproducible temperature increase.
To calibrate the temperature increase and its distribution in one cell culture dish for each power setting, the temperature was measured during 10 s of irradiation at different positions and at the cellular level. The temperature distribution was represented using a mathematical graphics software program, and its pattern across the culture dish was in Gaussian form. After laser irradiation, different biological experiments could be performed to assess temperature-dependent cell responses. In this manuscript, viability staining (i.e., distinguishing live, apoptotic, and dead cells) is introduced to help determine the threshold temperatures for cell apoptosis and death after different points in time.
The advantages of this method are the preciseness of the temperature and the time of heating, as well as its high efficiency in heating cells in a whole cell culture dish. Furthermore, it allows for study with a wide variety of temperatures and time durations, which can be well-controlled by a computerized operating system.
Introduction
Understanding temperature-dependent cell biological responses is of great importance to successful hyperthermia treatments. Retinal laser photocoagulation with a thermal laser, used in ophthalmology, is one of the most established laser treatments in medicine. Visible light, mostly from green to yellow wavelengths, is used in retinal laser treatment. The light is highly absorbed by the melanin in retinal pigment epithelial (RPE) cells, which form the outermost cell monolayer of the retina. There has been recent interest among physicians and researchers in very mild thermal irradiation (sub-visible photocoagulation) as a new therapeutic strategy for different kinds of retinal disorders1,2. Following this trend, our interest is in sub-lethally heating RPE cells under precise temperature control, a technique called temperature-controlled photothermal therapy (TC-PTT).
Recent optoacoustic technology from our institute has allowed for the real-time measurement of temperature increases at irradiated sites in the retina. This enables control over the temperature increase during irradiation3. However, since sub-lethal hyperthermia on the retina, caused by heating RPE cells sub-lethally, has not been previously considered due to the impossibility of measuring and controlling the temperature, the temperature-dependent cell responses of RPE cells following thermal laser irradiation has been studied very little to date. Moreover, not only has the temperature difference not been discussed in detail, but also the difference in the cell behavior of the surviving cells after sub-lethal and lethal irradiation. Therefore, to gather scientific evidence on TC-PTT-based treatments, we aim to elucidate the temperature-dependent RPE cell biological responses and their mechanisms using in vitro experimental setups.
For this purpose, it is necessary to establish a cell-heating setup that meets the following conditions: 1) a possibility for fast temperature increases, 2) a precisely controlled time and temperature, and 3) a relatively high number of examined cells for biological experiments. Regarding the heating method, a clinical laser, such as a frequency-doubled Nd.YAG laser (532 nm), is unfortunately unsuitable for cell culture heating. This is because of the strongly reduced number of melanosomes in cultured RPE cells. The laser light absorption might be inhomogeneous, and the temperature increase at the cellular level is variable between experiments, even when irradiated with same radiation power. Several previous studies have reported the use of black paper beneath the dish bottom during irradiation4 or the use of additional melanosomes that are phagocytized by the culture cells before the experiments5,6. Many of the in vitro biological studies to assess hyperthermia-induced cell responses have been performed using a hot plate, a water bath, or a CO2 incubator with a temperature setting7. These methods require a long heating period because it takes some time (i.e., several minutes) to reach the desired temperature. Furthermore, using these methods, it is difficult to obtain a detailed thermal history (i.e., temperature multiplied by time) at the cellular level. Moreover, the temperature among the cells at different positions in one culture dish may differ due to variable temperature diffusion. In most cases, this temporal and spatial temperature information during hyperthermia has not been taken into consideration for biological analyses, even though biological cell response may be critically affected by the temperature and the time duration of the increased temperature.
To overcome these problems, a continuous-wave thulium laser was used here to heat the cells. Thulium laser radiation (λ = 1.94 µm) is strongly absorbed by water8, and the cells at the bottom of the culture dish are thermally stimulated solely through thermal diffusion. The laser fiber with a 365-µm diameter is set about 12 cm above the culture dish, without any optics in between. The laser beam diameter diverges such that it is almost equivalent to the inner diameter of the culture dish (30 mm) at the surface of the culture medium.With a consistent amount of culture medium, it is possible to irradiate the cells with the temperature increase of high repeatability. Variable power settings enable irradiation with up to 20 W, and the medium temperature at the cellular level may be increased up to ΔT ≈ 26 °C in 10 s.
By modifying the irradiation conditions, it is also possible to change the laser beam profile to vary the temperature distribution in a culture dish. For example, it is possible to investigate with a Gaussian-like temperature distribution, as in the current study, or with a homogeneous temperature distribution. The latter may be advantageous for investigating the effects of temperature-dependent cell responses more specifically for sub-lethal temperature increases, but not for cell death stress or wound healing responses.
Altogether, thulium laser irradiation may enable the investigation of different kinds of biological factors, such as gene/protein expression, cell death kinetics, cell proliferation, and cell functionality development, after different thermal exposures.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
1. RPE Cell Culture
- Isolation of RPE cells from porcine eyes
- Obtain freshly enucleated porcine eyes from the local slaughterhouse. Keep them cool (4 °C) and in a dark environment.
- Remove extracellular tissues with scissors and soak the eyes in an antiseptic solution for 5 min.
- Place the eyes in sterilized phosphate-buffered saline without calcium and magnesium (PBS (-)) until use.
- Using a scalpel, penetrate the sclera at about 5 mm posterior to the corneal limbus. Resect the whole anterior part of the eye by cutting with scissors all way through, parallel to the corneal limbus.
- Remove the anterior part of the eye (i.e., the cornea and lens) and the vitreous. Add 1 mL of PBS (-) and gently remove the neural retina.
NOTE: This "eye cup," consisting of the sclera, choroid, and RPE, is now ready. - Add pre-warmed (37 °C) 0.25% trypsin in PBS (-) to the eye cup. Adjust the volume such that about 80% of the eye cup is filled with this trypsin solution.
- Incubate the eye cup with the trypsin solution in a 5% CO2 incubator at 37 °C for 10 min.
- Remove the eye cup from the incubator and replace the 0.25% trypsin solution with a PBS (-) solution with 0.05% trypsin + 0.2% ethylenediaminetetraacetic acid tetrasodium salt (EDTA·4N). Incubate the eye cup in the incubator for 45 min.
NOTE: After 45 min, the RPE cells will be either just loosely attached to the Bruch's membrane or already detached and floating in the trypsin-EDTA solution. - Collect the RPE cells by gentle pipetting. Collect the cells and solution in a conical tube filled with 10 mL of culture medium (DMEM high glucose with L-glutamine), including 10% porcine serum, antibiotic/antimycotic, and sodium pyruvate (1 mM).
NOTE: The serum may neutralize the effect of trypsin. - Centrifuge the cell suspension at 400 x g for 5 min at room temperature.
- Remove the supernatant and add 10 mL of fresh medium. Centrifuge again under the same conditions for 5 min.
- Remove the supernatant and add new medium, such that the cell concentration results in 5 x 105cells/mL (determined by counting the cells using a hemocytometer). Mix well by gentle pipetting.
- Distribute the cell suspension in cell culture dishes. Use 3 mL per 60 mm-diameter culture dish.
NOTE: This culture is called passage zero (P0). - Maintain the cells in a 5% CO2 incubator at 37 °C. Change half of the conditioned medium to fresh medium every second day.
- Subculture (step 1.2) if it becomes confluent.
- Subculture of the RPE cell culture
- Remove the culture medium and rinse the cells twice with PBS (-).
- Incubate the cells with PBS (-) solution with 0.05% trypsin + 0.2% EDTA in a 5% CO2 incubator at 37 °C for 5 min.
- Detach the RPE cells by gentle pipetting and collect the cell suspension in a conical tube filled with 10 mL of culture medium, including 10% porcine serum.
- Centrifuge the cell suspension at 400 x g for 5 min at room temperature.
- Remove the supernatant and add new culture medium, making the cell concentration 5 x 105 cells/mL (determined by counting the cell number with a hemocytometer). Distribute the cells in new 60 mm-diameter culture dishes, as described in step 1.1.13.
NOTE: The cell culture is now passage 1 (P1). - After confluency is reached, subculture the P1 culture to P2, using the same procedure described in steps 1.2.1-1.2.5. From the P2 culture, seed the cells on smaller culture dishes (30-mm inner diameter) instead of 60 mm-diameter culture dishes.
- For the experiments, use P2 or P3 cultures.
2. Thulium Laser Irradiation
- Construction of the irradiation station
- Connect a thulium laser device (1.94 µm, power range: 0-20 W) to a 0.22-NA, 365-µm core diameter fiber.
- Mechanically fix the fiber tip to the metal arm that is horizontally fixed to the vertical metal post of the irradiation station. Place the vertical post such that the tip of the laser fiber is located above the hot plate on which the cell culture dish is to be placed during irradiation.
- Lay a white paper on the hot plate and turn the aiming beam on (λ = 635 nm, max = 1 mW, diameter at paper level ≈ 30 mm). Mark the circumference of the aiming beam on the white paper so that the position where the culture dish is to be placed during irradiation is known.
NOTE: The z-plane of the fiber tip may be changeable. Without any additional imaging optics, the laser spot diameter on the cell culture plane, placed 12 cm below the fiber tip, is about 30 mm, which is almost equivalent to the inner diameter of the cell culture dish. A schematic drawing of the setup is shown in Figure 1.

Figure 1: Schematic Image of the Thulium Laser Irradiation Station. A culture dish is placed on the heating plate. The cells are placed 12 cm below the thulium laser fiber tip so that the beam size is almost identical to the inner diameter of the culture dish (about 30 mm). The laser irradiation procedure is controlled by a time-controlled routine of the custom-made system design platform. The power setting must be determined before the irradiation program is started. Please click here to view a larger version of this figure.
- Laser irradiation of the cell culture
- 1 h before irradiation, replace the culture medium completely with 1.2 mL of fresh medium.
NOTE: This is a CRITICAL STEP and must be strictly followed. - Place the irradiation station (i.e., the hot plate and the post with which to fix the laser fiber) on a clean bench.
- Remove the cell culture dish from the incubator and place it on the marked position on the hot plate (step 2.1.3).
- Wear protective glasses. Turn on the thulium laser. Set the power as desired on the laser device (tunable from 0 - 20 W). Turn the emission on.
- Start a system design platform that controls the laser irradiation and timing protocol (supplemental file).
- Immediately after placing the culture dish on the hot plate, click the "pre-heating time" to start the timer for 140 s ("pre-heating time 1"); this will keep the culture medium temperature at 37 °C before irradiation.
NOTE: After 140 s, a beep sound will turn on, and the next timer ("pre-heating time 2") will start automatically counting 8 s. During this 8 s, the examiner may open the culture dish. After 148 s of pre-heating, a 10 s-long laser irradiation on the cell culture will be conducted automatically. In case of emergency, equip the laser device with a force-quit button to stop the laser immediately. This is a CRITICAL STEP and must be strictly followed. A special point of caution relates to opening the cover of the dish just before irradiation, at the beginning of the 8 s pre-heating time. Opening the cover may cool down the medium surface very quickly. - After irradiation, immediately place the cover back onto the culture dish, leave the culture dish on the hot plate for an additional 7 s, and place it back in the 5% CO2 incubator at 37 °C.
- 1 h before irradiation, replace the culture medium completely with 1.2 mL of fresh medium.
- Measurement of the temperature distribution at the cellular level (temperature calibration)
- Make small holes (about 300 µm in diameter) close to the bottom on four sides (every 90°) of a 30 mm diameter culture dish (without cells); use the tip of a needle (20G) heated with a Bunsen burner. Seal the holes with electrical isolation tape from outside and make a small hole with a fine needle so that only a fine thermocouple (200 µm in diameter) may be inserted through this hole under watertight conditions.
- At the outside of the culture dish bottom, draw 2 perpendicular diameters and set the crossing point (i.e., the center of the bottom side) as the coordinate zero (0). Mark every 3 mm radially to the outside out the dish (i.e., 0, 3, 6, 9, 12, and 15 mm) in each direction along the lines (Figure 2, blue dots); the number of points should be 21 in total.
- Fill the cell culture dish with 1.2 mL of new culture medium. Place the culture dish onto a hot plate at 37 °C, insert a fine thermocouple (200 µm in diameter) into the side hole, and place its sensitive tip onto a marked position to be measured.
- Wear protective glasses. Turn on the thulium laser and manually set the power (between 0 and 20 W, in 0.1-W increments) of the laser devise.
NOTE: For temperature calibration, measurements with the power in increments of 3 W should be sufficient. - Turn on the system design platform and click the "Start Temp. Acquisition" button (supplemental file) to start the temperature measurement.
- Conduct same procedure as in step 2.2.6.
NOTE: The control program measures the temperature of the inserted thermocouple every 100 ms and shows the temperature progression during irradiation in the GUI. - Conduct these procedures for all 21 measurement points and at different power settings. Repeat the whole procedure three times for all points and for all power settings to achieve reliable data.
- Export temperature data as csv data, which can eventually be converted to a spreadsheet. Average the maximal temperature at the end of irradiation for the triplicate measurements at every point. Average the values from the points on the same circle (4 points in total, except the central point).
- Plot the obtained averaged maximal temperature on a graph, making the distance from the center of the dish (mm) as the x-axis and the temperature increase (ΔT, °C) as the y-axis. Use the fit function of a mathematical software program to fit a Gaussian model to the raw data. Create a Gaussian fit temperature distribution.

Figure 2: The Points for Temperature Calibration in One Cell Culture Dish. The temperature data was measured in the center and at 5 radial points over 4 different angles (blue dots). Please click here to view a larger version of this figure.
3. Biological Assessments for Cell Responses after Different Thermal Irradiations
- Assessment of cell viability ( i.e., living, apoptotic, and dead) following different power settings and the determination of the cell death threshold
- At the indicated time points (i.e., 3, 24, and 48 h after irradiation), wash the cells with PBS (-) and use a commercially available kit to assess cell viability (i.e., vital, apoptotic, dead) according to the manufacturer's protocol.
- Prepare a staining solution by adding 5 µL of fluorescein isothiocyanate (FITC)-annexin V, 5 µL of ethidium homodimer III, and 5 µL of Hoechst 33342 to 100 µL of 1x binding buffer (all are kit components). Prepare enough staining solution to cover the cells. Incubate the cells for 15 min.
- Wash the cell culture with the binding buffer twice, replace the binding buffer with PBS (-), and set the culture on the stage of a fluorescence microscope.
- Switch the light path to the ocular lens, select the 4',6-Diamidin-2-phenylindol (DAPI) filter, turn on the illumination light, and find the focused plane with the 4x objective.
- Change the light path to the camera, find the image on the computer screen in the microscope imaging software, and adjust the focus.
- Use the stitch function (i.e., the function to record multiple images across the dish and then create a single, large image) of the microscope-specific software to obtain the fluorescence image of the whole cell culture dish. Use 3 different filter sets-DAPI, FITC, and tetramethylrhodamine (TRITC)-to image Hoechst 33342-positive cells (all cell nuclei), FITC-annexin V-positive cells (apoptotic), and ethidium homodimer III-positive cells (dead), respectively.
- Measure the radius (mm) of the dead (ethidium homodimer III-positive) region and the outer/inner radius of the apoptotic (annexin V-positive) band-form region in the stained cell cultures. Apply these radii to the fitted Gaussian function of the temperature distribution for the corresponding power setting. Calculate the exact temperature at the rim of the dead or apoptotic region to clarify the threshold temperatures for cell death and apoptotic change.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Temperature distribution after different power settings
All temperature developments for each single irradiation were monitored in the temperature calibration. From this data, the maximal temperature at the measured point was obtained and defined as Tmax (°C). As shown in Figure 3A, the program was executed at the time point when the culture dish was placed on the heating plate. After the 140 s "pre-heating time 1," which was needed to archive a stable medium temperature at 37 °C, the cover of the culture dish was removed during the 8 s "pre-heating time 2." At the end of "pre-heating time 2," laser emission started automatically. This curve is a representative temperature progression for a 10 s irradiation. During irradiation, the temperature increased, and immediately after the laser emission was turned off, temperature started to decrease. The maximal temperature at the center of the culture dish was defined in this study as Tmax (°C). The Tmax was proportional to the laser power (Figure 3B). Figure 3C shows the distribution of the maximal temperature for each power across the culture dish. The distributions are bell-shaped, as shown in Figure 3C, and fit to a Gaussian function according to the following formula:
t(r)= tbase + A・
where r, tbase, A, and w stand for the distance from the center (mm), the lowest temperature for the curve, the amplitude, and the width of the curve, respectively. The parameters (tbase, A, and w) of the fitted Gaussian curve for each power setting, namely for each Tmax,are shown in the table next to the graph.
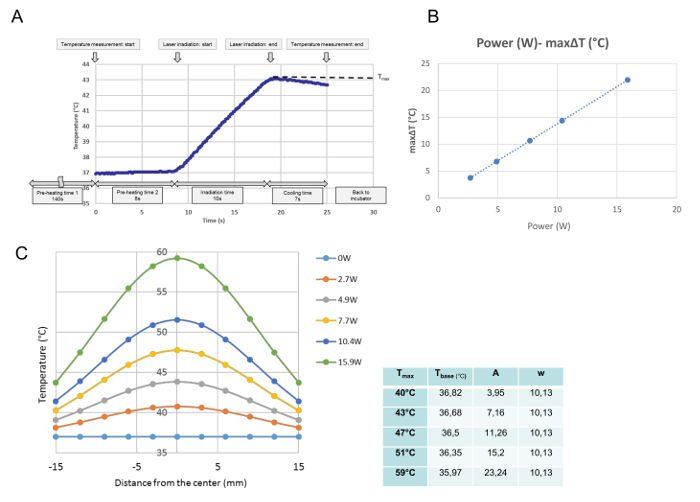
Figure 3: Temperature Calibration Data. A Representative Temperature Development at the Central Position after a Single Irradiation at 4.9 W (Tmax = 43°C) (A), the Proportional Relationship Between the Laser Power and the Maximal ΔT at the Central Position of a Cell Culture (B), and Temperature Distributions Across the Culture Dish after Different Power Settings (C). (A) The program is executed from the time point at which the culture dish is placed on the heating plate. After 140 s of "pre-heating time 1," which is needed to archive a stable medium temperature at 37 °C, the cover of the culture dish is removed for the 8-s "pre-heating time 2." At the end of "pre-heating time 2," the laser emission starts automatically. This curve is a representative temperature progression at the central position during a 10-s irradiation at 4.9 W. During irradiation, the temperature increases, and immediately after the laser emission is turned off, the temperature starts to decrease. The maximal temperature is obtained at the end of the irradiation, which is defined in this study as Tmax (°C). (B) The laser power and the maximal temperature increase (ΔTmax) are proportional. (C) The fitted Gaussian functions of the measured temperature distributions across the culture dish. The parameters for the functions, determined with a mathematical software program, are shown in the table at the side of the graph. Please click here to view a larger version of this figure.
Cell viability after thermal irradiation
As shown in Figure 4A, there are three different staining patterns indicating cell viability after laser irradiation: 1) no annexin V/ethidium homodimer III-positive (i.e., only live), 2) annexin V-positive at the center (i.e., almost only early apoptosis), and 3) ethidium homodimer III-positive at the center (i.e., dead cells) surrounded by apoptotic cells at the border between dead and live cells (Figure 4B). The size of the dead/apoptotic area is generally dependent on Tmax and the post-irradiation time up to 48 h after irradiation. No apparent viability change was detected in the cultures irradiated with Tmax≤43 °C. The only apoptotic change could be observed at an early point in time (3 h), followed by a late cell death after an irradiation with Tmax = 47 °C. Immediate or early cell death (up to 3 h) was found in the cultures irradiated with Tmax≥ 51 °C (Table 1).

Figure 4: Pattern of Viability Staining After Different Power Settings (A) and an Exemplary Image at the Rim of the Dead and Apoptotic Area after Lethal Laser Irradiations (B).
(A) Three patterns of staining can occur, depending on the temperature. (B) The apoptotic zone (FITC-annexin V-positive: green) around the dead area (ethidium homodimer III-positive: red). All cells are positive for Hoechst 33342 (blue), and the cells with blue nuclei are the live cells. The image was taken 24 h after an irradiation at Tmax= 59 °C. Bar = 100 µm. Please click here to view a larger version of this figure.

Table 1: Annexin V and Ethidium Homodimer III Responses at Various Temperatures and Times.
Determination of the threshold temperature for cell death
The average radii of the dead area (red) and the apoptotic area (green) were measured and applied to the Gaussian function of the temperature distribution to determine the threshold peak temperatures for cell death and apoptosis after 10 s of irradiation. According to this analysis, the mean threshold temperatures for complete cell death 3 h, 24 h, and 48 h after irradiation were 54.0 °C, 50.9 °C, and 50.1 °C, respectively. The mean threshold temperature for cell apoptotic change were lower by about 2 - 3 °C , with the threshold temperatures for 3 h, 24 h, and 48 h at 51.7 °C, 48.0 °C, and 47.0 °C, respectively (Figure 5).
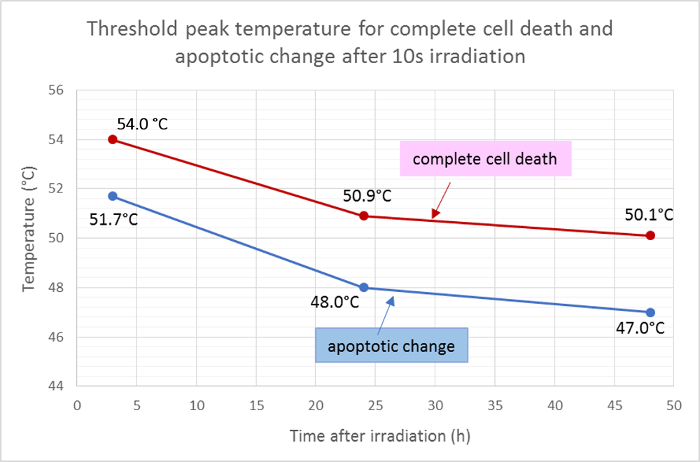
Figure 5: Threshold Temperatures for Apoptosis and Cell Death.
Mean threshold temperatures for complete cell death (positive for Hoechst 33342, annexin V, and ethidium homodimer III) and for apoptosis (positive only for Hoechst 33342 and annexin V) at different time points in after irradiation, calculated from the results of fluorescence viability staining. Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
In discussing temperature-related biological cellular responses, not only the temperature, but also the time duration of the increased temperature, is of importance, since most biochemical processes are time-dependent. Particularly in the field of laser-induced hyperthermia in ophthalmology, due to the short time range-from milliseconds to seconds-it is difficult to investigate cellular thermal effects with precise temperature control. Therefore, a laser irradiation setup suitable for the cell culture model and with an operation system that enables strict temperature and time control is desired. The biological assessment of cell responses after thermal exposure, such as protein expression or secretion, requires repeated quantitative evaluations on a sufficient number of affected cells. This has been an obstacle to studies using laser spots several hundred micrometers in diameter, as in clinical treatments. Quantitative analysis at a single laser spot is quite burdensome. In this study, attempts have been made to fulfill these demands as much as possible. By using a 1.94 µm wavelength continuous-wave thulium laser with an irradiation control program, a temporal temperature rise could be conducted in a whole cell culture within a short timeframe. Since the temperature distribution may be adjusted by changing the light path, different kinds of hyperthermia-related experiments can be conducted using this setup.
The limitation of the presented technique is the impossibility of conducting simultaneous temperature measurements during the laser irradiation of the cells. Since the use of thermocouples is not suitable for sterilized cell cultures, temperature calibration must be conducted separately from cell irradiation. Considering the possible variations in the laser-power output, real-time temperature measurements during each laser irradiation would be ideal to directly assess cellular responses corresponding to the thermal dose. Moreover, the temperature distribution used here was created through data interpolation based on the measurements at 21 points on a culture dish and at several different power settings. Therefore, to overcome these limitations and critical points, it is our goal to develop an alternative method that allows for the measurement of the temperature of the culture dish while laser irradiation is being performed. We also aim obtain the spatial temperature information at once. Infrared imaging (thermography) is one possible method to measure the temperature during laser irradiation10. The great advantage of this method is the real-time temperature measurement at the cellular level for each irradiation; subsequent cell biological responses can always be individually compared to the temperature history during irradiation. Considering cost-effectiveness and usability, however, using thermography for cell heating experiments is not possible for every laboratory.
In the method using a thulium laser at a wavelength of 1.94 µm, the water in the cell culture dish is heated at its surface, and thermal diffusion and convection are used to heat the cells. The height of the culture medium in this irradiation setup, with 1.2 mL of culture medium, is 935 µm at the central position (from a previous measurement using optical coherence tomography). The level of absorption of the thulium laser in water is very high (absorption coefficient: 127 cm-1 at 35 °C), and 72% of the light is absorbed in the first 100 µm of the culture medium. There is almost no absorption (0.0007%) at a depth of 935 µm.
It is important to note that one of the critical points in the protocol is to add the same amount of medium (1,200 µL) for each irradiation. Using different amounts of culture medium may lead to the height differences, which may cause differences in the temperature increase of the cells. The second critical point relates to the timing of the opening of the culture dish. It must be done at the same time-in this study, 8 s before the start of the irradiation, when the system makes a sound. Differences in this timing may vary the base temperature due to the cooling caused by the surrounding air (about 23 °C). This can lead to significant differences in the laser-induced temperature.
For temperature calibration, the same amount of medium (1.2 mL) that was used in the experiments was used to measure the temperature distribution at the bottom of cell-free culture dishes. However, the medium height with a cell monolayer may be different than the one without cells, even with the same volume of medium added. The measurement using optical coherence tomography revealed that there is a 58-µm difference at the central position between dishes with and without a confluent cell monolayer (877 µm without cells, compared to 935 µm with cells). This difference is potentially due to the capillary action of the cells. The 58-µm difference in height at the central position may be caused by approximately 40 µL of medium (measured data). It was also confirmed that this difference in height did not cause significant differences in Tmax at all power settings. Therefore, we have concluded that this difference does not significantly influence the results of the analyses done in this study. Nevertheless, to gather more precise temperature information, as written above, a method to calibrate the temperature using a cell culture dish containing a cell monolayer should be developed. Moreover, the mathematical modeling of thermal diffusion and convection in the whole culture medium is also required.
In this study, the cells were heated with a Gaussian temperature distribution. There are several possible methods to heat the whole medium more uniformly over time. One is to use a laser source with a lower absorption coefficient in water. However, the drawback is that, in this case, the lasers must have a higher power, since only a small percentage of the light is absorbed over about 0.9 mm. Another possibility is to image the distal fiber tip of a multimode optical fiber that transmits the laser light into the plane of the culture dish; the magnification can be chosen arbitrarily by the optics.
The second highlight of this protocol is its ability to determine the threshold temperature for cell death and apoptosis using the fluorescent image of viability staining and the lateral temperature distribution. A long-term aim is not only to determine cell viability, but also to elucidate the temperature range for cell biological responses relating to cell functionality, such as protein expression and cell proliferation. The determination of the cell death threshold temperature is of great interest to researchers10. Using this method, it might be possible to determine the critical factors for cell death, including apoptosis. Critical factors for thermal laser-induced cell death might be determined not only through the temperature history, but also through the endogenous factors (i.e., intra/extra-cellular factors at the molecular level). Answering these questions might pave the way for understanding cell death mechanisms and kinetics after different thermal exposures and in different retinal pathologies. Furthermore, it can also help to clarify clinically observed issues, such as the inter-individual difference in response to laser treatment or the variability in scar size after retinal photocoagulation, even when the initial spot size was almost identical ("atrophic creep")11.
The final aim of this study is to aid in developing temperature-controlled photothermal therapy of the retina. To achieve this, in parallel with the technical advancement of the temperature measurement3, further elucidation of RPE cell behavior after thermal exposure, determined using this method, will be of great benefit.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by a research grant from the German Federal Ministry of Education and Research (BMBF) (grant #13GW0043C) and and a European Office of Aerospace Research and Development (EOARD, grant # FA9550-15-1-0443)
Materials
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Dulbecco’s Modified Eagle’s Medium - high glucose | Sigma-Aldrich | D5796-500ML | Add (2)-(4) before use. Warm in 37 °C water bath before use. |
| Antibiotic Antimycotic Solution (100 ×) | Sigma-Aldrich | A5955-100ML | Containing 10,000 units penicillin, 10 mg streptomycin and 25 μg Amphotericin B in 1mL. Add 5.5 mL in 500 mL medium bottle (1) before use. |
| Sodium pyruvate (100 mM) | Sigma-Aldrich | S8636-100ML | Add 5.5 mL in 500 mL medium bottle (1) before use (final concentration: 1 mM) |
| Porcine serum | Sigma-Aldrich | 12736C-500ML | Add 50 mL in 500 mL medium bottole (1) before use (final: 10%) |
| Phosphate Buffered Saline (PBS) | Sigma-Aldrich | D8537-500ML | |
| Trypsin from porcine pancreas | Sigma-Aldrich | T4799-25G | |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | ED-100G | |
| Human VEGF Quantikine ELISA Kit | R&D System | DVE00 | |
| Oxiselect Total Glutathione Assay Kit | Cell Biolabs, Inc | STA-312 | |
| Apoptotic/Necrotic/Healthy Cells Detection Kit | PromoKine | PK-CA707-30018 | |
| Name | Company | Catalog Number | Comments |
| Equipments | |||
| Thulium laser | Starmedtec GmbH | Prototype | 0-20 W |
| 365 mm core diameter fiber | LASER COMPONENTS Germany | CF01493-52 | |
| Thermocouple | Omega Engineering Inc | HYP-0- 33-1-T-G-60-SMPW-M | |
| Heating plate | MEDAX | ||
| Microplate reader (spectrofluorometer) | Molecular Device | Spectramax M4 | |
| cell homogenizer | QIAGEN | TissueLyser LT | |
| Fluorescence microscope | Nikon | ECLIPSE Ti | |
| mathematical software program | The Mathworks. Inc | MATLAB Release 2015b | |
| system-design platform | National Instrument | Labview | Laboratory Virtual Instrument Engineering Workbench |
References
- Inagaki, K., et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561-577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 59 (1), 21-28 (2015).
- Roider, J., et al. Selective retina therapy (SRT) for clinically significant diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 248 (9), 1263-1272 (2010).
- Brinkmann, R., et al. Real-time temperature determination during retinal photocoagulation on patients. J Biomed Opt. 17 (6), 061219 (2012).
- Yoshimura, N., et al. Photocoagulated human retinal pigment epithelial cells produce an inhibitor of vascular endothelial cell proliferation. Invest Ophthalmol Vis Sci. 36 (8), 1686-1691 (1995).
- Denton, M. L., et al. Damage Thresholds for Exposure to NIR and Blue Lasers in an In Vitro RPE Cell System. Invest Ophthalmol Vis Sci. 47 (7), 3065-3073 (2006).
- Shrestha, R., Choi, T. Y., Chang, W., Kim, D. A high-precision micropipette sensor for cellular-level real-time thermal characterization. Sensors (Basel). 11 (9), 8826-8835 (2011).
- Gao, F., Ye, Y., Zhang, Y., Yang, J. Water bath hyperthermia reduces stemness of colon cancer cells. Clin Biochem. 46 (16-17), 1747-1750 (2013).
- Jansen, E. D., van Leeuwen, T. G., Motamedi, M., Borst, C., Welch, A. J. Temperature dependence of the absorption coefficient of water for midinfrared laser radiation. Lasers Surg Med. 14 (3), 258-268 (1994).
- Iwami, H., Pruessner, J., Shiraki, K., Brinkmann, R., Miura, Y. Protective effect of a laser-induced sub-lethal temperature rise on RPE cells from oxidative stress. Exp Eye Res. 124, 37-47 (2014).
- Denton, M. L., et al. Spatially correlated microthermography maps threshold temperature in laser-induced damage. J Biomed Optics. 16 (3), (2011).
- Morgan, C. M., Schatz, H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 96 (1), 96-103 (1989).



