Summary
We present a protocol to efficiently evaluate aneurysm perfusion and vessel patency of sidewall aneurysm in rats and rabbits, using fluorescein-based fluorescence video angiography (FVA). With a positive predictive value of 92.6%, it is a simple but very effective and economical method with no special equipment required.
Abstract
Brain aneurysm treatment focuses on achieving complete occlusion, as well as preserving blood flow in the parent artery. Fluorescein sodium and indocyanine green are used to enable the observation of blood flow and vessel perfusion status, respectively. The aim of this study is to apply FVA to verify real-time blood flow, vessel perfusion status and occlusion of aneurysms after induction of sidewall aneurysms in rabbits and rats, as well as to validate the procedure in these species.
Twenty sidewall aneurysms were created in 10 rabbits by suturing a decellularized arterial vessel pouch on the carotid artery of a donor rabbit. In addition, 48 microsurgical sidewall aneurysms were created in 48 rats. During follow-up at one month after creation, the parent artery/aneurysm complex was dissected and FVA was performed using an intravenous fluorescein (10%, 1 mL) injection via an ear vein catheterization in rabbits and a femoral vein catherization in rats. Aneurysms were then harvested, and patency was evaluated macroscopically.
Macroscopically, 14 out of 16 aneurysms in rabbits indicated no residual parent artery perfusion with totally occluded luminae, however 11 (79%) were detected by FVA. Four aneurysms were excluded due to technical problems. In rats, residual aneurysm perfusion was macroscopically observed in 25 out of 48 cases. Of the 23 without macroscopic evidence of perfusion, FVA confirmed the incidence of 22 aneurysms (96%). There were no adverse events associated with FVA. Fluorescein is easily applicable and no special equipment is needed. It is a safe and extremely effective method for evaluating parent artery integrity and aneurysm patency/residual perfusion in an experimental setting with rabbits and rats. FVA using fluorescein as a contrast agent appears to be effective in controlling patency of aneurysms and the underlying vessel and can even be adapted to bypass surgery.
Introduction
Evidence of complete aneurysm obliteration and parent artery integrity is of utmost importance in aneurysm surgery. There are several options to confirm parent artery patency and aneurysm occlusion, such as Doppler sonography, conventional cerebral angiography (DSA), computed tomography angiography (CTA) or magnetic resonance angiography (MRA)1,2. However, these are expensive and time-consuming methods which are often not available in a laboratory setting. Furthermore, they may have relevant side effects such as radiation exposure or need for additional sedation of experimental animals to avoid movement artefact.
With an increasing number of new endovascular devices emerging, there is a consecutive need for preclinical testing of such devices. However, these studies often rely on post-mortem analysis (e.g., macro pathology and histology) and lack information on dynamic perfusion. Furthermore, for the researcher it may be crucial to obtain immediate and reliable information during an experimental surgical procedure. Fluorescence angiography is a cost-effective and easy to perform visualization technique1,3,4.
As such, indocyanine green (ICG) video angiography is often used in clinical neurosurgical procedures and has extensively been studied5,6. Fluorescein video angiography (FVA) is an alternative technique, with the additional advantage of creating a fluorescence signal that is within the wavelength range of human vision, and can thus be seen by the naked eye without an extended spectrum infrared camera7. Fluorescein video angiography is less often used in clinical cerebrovascular surgery and reports on FVA in experimental settings are scarce1,4.
The aim of this report is to demonstrate the feasibility and scope of applications of FVA in rat and rabbit preclinical cerebrovascular research.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
The rodents were housed in an animal care facility and experiments were reviewed and approved by the Committee for Animal Welfare at the University of Bern, Switzerland (BE 108/16) and (BE65/16). All animals were maintained on a standard laboratory diet with free access to food and water. All animal experiments were conducted under careful consideration of the 3Rs (replacement, reduction and refinement). Ten female New Zealand White rabbits and 48 male Wistar rats were included. ARRIVE guidelines were followed strictly8.
NOTE: Twenty sidewall aneurysms were created in 10 rabbits by suturing a decellularized arterial vessel pouch on the carotid artery of a donor rabbit. In addition, 48 microsurgical sidewall aneurysms were created in 48 rats as described before4,9. The imaging procedure and macroscopic analysis was performed 4 weeks after aneurysm creation.
1. Preparation of material needed for fluorescein video angiography
- Modify the flashlight by taping on a blue bandpass filter (see the Table of Materials), which will function as an excitation filter. The torch should then only emit blue light. Use black tape to avoid any leakage of unfiltered light.
- Equip the camera (e.g., attached to the microscope) with a green bandpass filter (see the Table of Materials), which will function as a emission light filter. Only green light should now be able to pass through.
2. Preparation of workplace and materials
- Disinfectant the workspace with disinfectant solution.
- Cover the table with sterile drapes to prevent contamination.
- Use sterile instruments for the surgery.
3. Preparation of animals for the surgery
- Weigh the animals.
- Induce anaesthesia and adjust the dose according to the weight.
- For rabbits, start balanced anesthesia. Shield their eyes with one hand during injection to reduce their fright reaction. Cover the cage with a sheet to help sedate the animals.
- Anesthetize rats in a gas chamber with 4% isoflurane and 96% oxygen prior to the injection.
- Monitor the depth of anaesthesia. Pinch between their toes to make sure the animals are asleep.
- Reposition the rabbits onto their backs. They should not react.
- For rats, pinch their tails and ensure that no reaction is observed.
- Apply ointment on the rodents' eyes to prevent dryness. Pull out the rats' tongues to avoid any chance of swallowing.
- Start with preservation of anaesthesia.
- For rabbits, catheterize (22 G shielded IV catheter with injection port, see the Table of Materials) the ear vein. Maintain balanced anaesthesia. Use a three-way stopcock to enable multiple simultaneous injections.
- For rats, inject 50 mg/kg ketamine hydrochloride and 0.5 mg/kg medetomidine hydrochloride intraperitoneally. Monitor anaesthesia with a noxious toe pinch during surgery. In the case of reaction, administer additional anaesthetic.
- Tape the animals onto the board in a supine position and closely shave the incision location. Disinfect the area with Betaseptic.
- For rabbits, disinfect the neck, especially around sternocleidomastoid muscle.
- For rats, disinfect the area from bladder to transvers colon.
- Administer oxygen through a mask throughout the surgery and maintain body temperature with a heating pad.
4. Preparation of the artery
- For best results, thoroughly dissect the chosen vessel from the surrounding tissue9,10.
- For rats, identify the tail vein (less invasive, preferably used for surviving animals) or dissect a femoral vein for fluorescein injection4,11.
NOTE: For rabbits, no further dissection of vessels is needed for fluorescein injection as the ear vein is already being used for anaesthesia.
- For rats, identify the tail vein (less invasive, preferably used for surviving animals) or dissect a femoral vein for fluorescein injection4,11.
- Position a white pad under chosen vessel to increase contrast with the surrounding tissue.
- Focus the camera mounted to the microscope on the dissected artery.
5. Fluorescein video angiography
- Cover the 5 mL syringe filled with fluorescein sodium (100 mg/mL, see the Table of Materials) with aluminium foil to protect from exposure of light. Turn off the lights (as much as possible) and inject fluorescein sodium intravenously. Inject under darkness to prevent photobleaching.
- For rabbits, inject 0.3 mL/kg fluorescein sodium through the three-way-stopcock into the catheterized ear vein.
- For rats, inject 0.4 mL/kg fluorescein sodium into the femoral vein via a catheter or a 25 G needle.
- Flush the needle or the catheter with 0.5 mL saline solution to ensure that all dye is injected.
- Illuminate the surgical field with the modified flashlight.
- Commence filming with the modified camera. Blood flow should be visible a few seconds after injection (Figure 1).
NOTE: Here, we used frame rate = 50 frames/s, focal length = 70 mm, and F3.4.
6. Macroscopic analysis
- Resect the aneurysms and parent artery complex, and evaluate the patency macroscopically by opening the parent artery with micro-scissors and evaluate the lumen of the parent artery and the anerysm’s orifice (see Figure 1, 2)9. Measure the sizes of the aneurysms. Aneurysm-parent-artery-complex can then be stored for further analysis (e.g., histology).
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Heart rate and blood pressure were monitored during surgery. Mean heart rate was 193/min in rabbits and 196/min in rats. The rabbits' body weight ranged 3.05-4.18 kg, and the rats weighed 335-690 g.
We were able to perform FVA in eight out of ten rabbits (Figure 1). Four aneurysm examinations in two rabbits were not recorded with the camera due to technical difficulties. No technical difficulties involving FVA in rats were reported. However, FVA could not be performed in one rat due to difficulties puncturing the femoral vein.
Of 16 aneurysms in eight rabbits, two aneurysms showed persistent perfusion of the parent artery (confirmed macroscopically) (see Table 1) while FVA identified five cases with residual perfusion. 14 rabbit aneurysms showed no residual perfusion macroscopically, however 11 (79%) were subsequently detected using FVA. Residual perfusion was observed macroscopically in 25 of 48 rats (Table 1), and the other 23 rats showed no macroscopic signs of residual perfusion (Figure 2). 22 of those 23 aneurysms were then confirmed using FVA (96%). Altogether, 25 of 27 cases could be confirmed, resulting in a positive predictive value of 92.6%, a sensitivity rate of 100% and specificity of 94.1%. (Table 2).
In summary, 25 aneurysms showed residual perfusion, 53 parent arteries were patent and 11 were occluded as confirmed macroscopically and on video angiography. There were only minor complications associated with FVA in rabbits; such as perforation of marginal ear vein during catheterization. No further adverse events were experienced. No mortality and no morbidity due to FVA was reported.
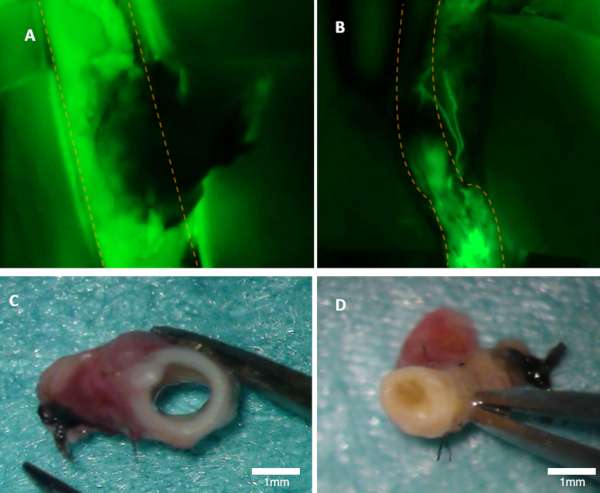
Figure 1: Visualizing patency in a rabbit. (A) Patency of the parent artery is clearly visible on the fluorescence image (green emission from fluorescein are seen). (B) This artery is occluded (fluorescence image). Both arteries were inspected macroscopically (C-D). Panel (C) shows in the artery from panel A; the lumen is open. Panel (D) shows artery from panel B where occlusion can be seen macroscopically. Orange dotted lines mark the borders of the parent artery. Please click here to view a larger version of this figure.

Figure 2: Visualizing perfusion in a rat. (A) This panel shows a residually perfused aneurysm (red dotted line marks the residual perfusion). (B) No perfusion can be detected. Panel (C) shows the artery from panel A during macroscopic scrutiny; the aneurysm orifice is open. (D) Macroscopic view of the neointima on an occluded aneurysm. Orange dotted lines mark the parent artery and the aneurysm’ dome. Panels (A) and (B) are fluorescence-only images and the green color shows fluorescein emission. Please click here to view a larger version of this figure.
| Patency/Residual Perfusion | ||||
| Macroscopical + | Macroscopical - | Fluorescein + | Fluorescein - | |
| Rabbits | 2 | 14 | 5 | 11 |
| Rats | 23 | 25 | 22 | 21 |
| Total | 25 | 39 | 27 | 32 |
Table 1: Patency testing. Patency of parent artery was only tested in rabbits and is illustrated here. Fluorescein detected more patencies of parent arteries than macroscopical evaluation. (All rats in this setting had an open parent artery, as aneurysms were sutured on the abdominal aorta.) The patency of aneurysms was tested in rats only. Twenty-two of 23 macroscopically detected patencies were confirmed using FVA. Twenty-one of 25 showed no patency on FVA.
| Macroscopic positive | Macroscopic negative | total | |
| Fluorescein positive | 25 | 2 | 27 |
| Fluorescein negative | 0 | 32 | 32 |
| total | 25 | 34 |
Table 2: Two-by-two table used to calculate specificity and sensitivity of FVA.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
FVA is a promising and uncomplicated method to examine vessels in rodents and can be performed with commercial devices and off-the-shelf equipment. FVA can be implemented during any surgery where intraoperative evaluation of vessel integrity is needed as the vessels need proper dissection first.
The authors preferred venous injection to arterial injection due to the lower risk of inadvertent events such as infection, ischemia and compartment syndrome12. Intravenous injection enables dependable, spatially limited, highly concentrated staining, and requires small dye dosages13,14. Additionally, venous injection allows a quick clearance of fluorescein14,15. An alternative method is to inject contrast agent directly into the chosen artery. This method was not used in these experiments as the investigators wanted to prevent contaminating the surgical field with blood and fluorescein. In order to reduce this risk, peripheral venous contrast agent injection is recommended13.
Advantages of FVA are high contrast (easily detectable with the human eye), high sensitivity as shown above (Table 2), low cost and easy handling16. Fluorescein sodium was the chosen contrast agent to examine perfusion. Visible light alone can be used for excitation of the dye and emission of the typical green light. Nevertheless, this contrast agent works best with blue light (approximately 480 nm) and emits a strong green light (wavelength approximately 530 nm)15. According to Yoshioka et al., fluorescein colors the artery extremely quickly14. Furthermore, the flow of fluorescein-enriched blood can be observed in real time15,17. The short time needed for FVA presents another advantage; in this series it took an average of 2 min (± 1min) to conduct one FVA.
The disadvantage of using fluorescein as a contrast agent is that it works well with only thin artery walls which demand very careful dissection. Ichikawa et al. showed the extinction of dye due to the thwarted emission of light through thicker walls by calcification or undissected arteries15. After injection, fluorescein is metabolised to fluorescent fluorescein glucuronide in the liver. Within 30 min after injection, the concentration of fluorescein glucuronide exceeds the concentration of fluorescein18. Fluorescein requires a long clearance time. An immediate reinjection after intravenous injection of fluorescein is not recommended as the artery and aneurysm are already fluorescent from the first injection17.
The molecular weight of fluorescein is only 376 kDa which allows leakage of the dye. The vascular wall also becomes fluorescent which could lead to false positive flow evaluations (increasing with time after application). A patchy coloration of the vessel wall was observed starting approximately 5 min after injection of fluorescein14. The spotty coloration, however, was only observed in larger arteries. Small and medium arteries did not show this staining structure17. It is recommended to evaluate the aneurysm immediately in order to detect residual filling.
Although there is a very low risk of toxicity, some cases of fluorescein leading to cardiac and respiratory reactions have been described14. In this study no severe adverse event occurred; the only complications were 2 cases of ear vein perforation. According to Lane et al., sodium fluorescein is not harmful even when used in humans17. On the other hand, fluorescein is quite unstable and should not be exposed to white light16 - a red light source can be used instead.
In order to choose the concentration of fluorescein for rabbits, the investigators started with the lowest known working dose in rats (0.2 mL of 100 mg/mL fluorescein sodium) and increased it gradually to 1 mL. A strong fluorescence signal was registered at that dose. The dosage was increased gradually to test if the fluorescence improves - which was not the case. The authors decided to continue with 1 mL of 100 mg/mL fluorescein sodium13.
Another dye available to examine vessels intraoperatively is ICG. Its size is 775 kDa and such barely penetrates the surrounding tissues14. Because of its longer emission wavelengths, tissue is penetrated more easily because tissues are more transparent at 800 nm19 and deeper structures become visible14,16. Excitation wavelength within the 750-800 nm are required16,20 and the emission wavelength from the contrast agent is approximately 800 nm16, making both invisible to the human eye. Due to its short half-life time in blood plasma, the dye can be injected and reused repeatedly16. Limitations to using this dye include problems with thick-walled arteries20 and the necessity of light outside the visible spectrum13. As a consequence, ICG is dependent on expensive equipment and not readily applicable in every laboratory.
In conclusion, FVA is a fast, inexpensive and reliable method with high sensitivity to screen patency of aneurysms and parent arteries in rodent aneurysm models. It is associated with virtually no morbidity and mortality. It allows real-time blood flow monitoring during surgery and follow-up. To improve its efficacy, the injection should be carried out in the dark and is best performed on meticulously dissected vessels. This method can easily and safely be implemented in a cerebrovascular laboratory, and can minimize experiment costs.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
All authors confirm no conflicts of interest.
Acknowledgments
This study was supported in part by a research grant from the Kantonsspital Aarau, Switzerland.
Materials
| Name | Company | Catalog Number | Comments |
| For rabbits | |||
| Aluminium foil | |||
| Animal shaver | |||
| Black tape | |||
| Blue filter | Thorlabs MF475-35 | ||
| Body warm plate | |||
| Camera | Sony NEX-5R | ||
| Catheter | 22G Vasofix Safety | ||
| Disinfictant | |||
| Fluorescein sodium | Fluorescein Faure 10% | ||
| Glas plate | |||
| Green filter | Thorlabs MF539-43 | ||
| Incontinence pad | |||
| Infusion pump | Perfusor Secura | ||
| Ketamine hydrochloride | any generic products | ||
| Needle | 25G | ||
| Oxygen | |||
| Ringer's Solution | |||
| Sterile sheets | |||
| Surgical instruments | micro forceps, micro scissor, blunt surgical scissor | ||
| Surgical microscope | OPMI, Carl Zeiss AG, Oberkochen, Germany | ||
| Syringe 2ml, 5ml, 50ml | |||
| Tape | |||
| Three-way-stopcock | |||
| Torch light | |||
| Xylazin | any generic products | ||
| For rats | |||
| Aluminium foil | |||
| Animal shaver | |||
| Black tape | |||
| Blue filter | Thorlabs MF475-35 | ||
| Body warm plate | |||
| Camera | Sony NEX-5R | ||
| Disinfictant | |||
| Fluorescein sodium | Fluorescein Faure 10% | ||
| Green filter | Thorlabs MF539-43 | ||
| Incontinence pad | |||
| Isoflurane | |||
| Ketamine hydrochloride | any generic products | ||
| Medetomidine hydrochloride | any generic products | ||
| Needle | 25G | ||
| Oxygen | |||
| Plate | |||
| Ringer's Solution | |||
| Sterile sheets | |||
| Surgical instruments | micro forceps, micro scissor, blunt surgical scissor | ||
| Surgical microscope | OPMI, Carl Zeiss AG, Oberkochen, Germany | ||
| Syringe 2ml, 5ml | |||
| Tape | |||
| Torch light |
References
- Kakucs, C., Florian, I. A., Ungureanu, G., Florian, I. S. Fluorescein Angiography in Intracranial Aneurysm Surgery: A Helpful Method to Evaluate the Security of Clipping and Observe Blood Flow. World Neurosurgery. 105, 406-411 (2017).
- Ajiboye, N., Chalouhi, N., Starke, R. M., Zanaty, M., Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. ScientificWorldJournal. 2015, 954954 (2015).
- Suzuki, K., et al. Confirmation of blood flow in perforating arteries using fluorescein cerebral angiography during aneurysm surgery. Journal of Neurosurgery. 107 (1), 68-73 (2007).
- Gruter, B. E., et al. Fluorescence Video Angiography for Evaluation of Dynamic Perfusion Status in an Aneurysm Preclinical Experimental Setting. Operative Neurosurgery. , Hagerstown. (2019).
- Raabe, A., et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. Journal of Neurosurgery. 103 (6), 982-989 (2005).
- Riva, M., Amin-Hanjani, S., Giussani, C., De Witte, O., Bruneau, M. Indocyanine Green Videoangiography in Aneurysm Surgery: Systematic Review and Meta-Analysis. Neurosurgery. , (2017).
- Kuroda, K., et al. Intra-arterial injection fluorescein videoangiography in aneurysm surgery. Neurosurgery. 72, 2 Suppl Operative 141-150 (2013).
- Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biology. 8 (6), 1000412 (2010).
- Marbacher, S., et al. The Helsinki rat microsurgical sidewall aneurysm model. Journal of Visualized Experiments. (92), e51071 (2014).
- Marbacher, S., et al. Complex bilobular, bisaccular, and broad-neck microsurgical aneurysm formation in the rabbit bifurcation model for the study of upcoming endovascular techniques. American Journal of Neuroradiology. 32 (4), 772-777 (2011).
- Shurey, S., et al. The rat model in microsurgery education: classical exercises and new horizons. Archives of Plastic Surgery. 41 (3), 201-208 (2014).
- Foster, S. D., Lyons, M. S., Runyan, C. M., Otten, E. J. A mimic of soft tissue infection: intra-arterial injection drug use producing hand swelling and digital ischemia. World Journal of Emergency Medicine. 6 (3), 233-236 (2015).
- Flower, R. W. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Investigative Ophthalmology & Visual Science. 12 (12), 881-895 (1973).
- Yoshioka, H., et al. Advantage of microscope integrated for both indocyanine green and fluorescein videoangiography on aneurysmal surgery: case report. Neurologia medico-chirurgica (Tokyo). 54 (3), 192-195 (2014).
- Ichikawa, T., et al. Development of and Clinical Experience with a Simple Device for Performing Intraoperative Fluorescein Fluorescence Cerebral Angiography: Technical Notes. Neurologia medico-chirurgica. 56 (3), 141-149 (2016).
- Alander, J. T., et al. A review of indocyanine green fluorescent imaging in surgery. International Journal of Biomedical Imaging. 2012, 940585 (2012).
- Lane, B., Bohnstedt, B. N., Cohen-Gadol, A. A. A prospective comparative study of microscope-integrated intraoperative fluorescein and indocyanine videoangiography for clip ligation of complex cerebral aneurysms. Journal of Neurosurgery. 122 (3), 618-626 (2015).
- Blair, N. P., Evans, M. A., Lesar, T. S., Zeimer, R. C. Fluorescein and fluorescein glucuronide pharmacokinetics after intravenous injection. Investigative Ophthalmology & Visual Science. 27 (7), 1107-1114 (1986).
- Hillmann, D., et al. In vivo optical imaging of physiological responses to photostimulation in human photoreceptors. Proceedings of the National Academy of Sciences of the United States of America. 113 (46), 13138-13143 (2016).
- Golby, A. J. Image-Guided Neurosurgery. , Elsevier Science. (2015).



