Overview
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Sublimation, the direct phase transition of a solid into a gas without first becoming a liquid, takes place at temperatures and pressures lower than that of the compound's triple point (Figure 1).The process of sublimation can be utilized to purify both organic and inorganic solids. During the purification technique, a solid is heated directly into the gas-phase. All non-volatile impurities are left behind while the vaporized compound is then collected (deposition) as a solid on a cold surface. Here, we will use sublimation to purify ferrocene, an inorganic solid with a triple point temperature of 183 °C.1
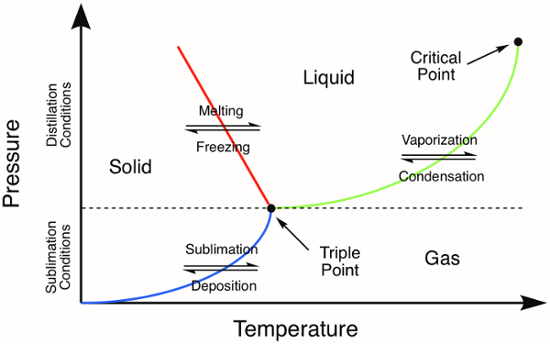
Figure 1. Generic phase diagram. The colored lines represent the pressure and temperature requirements for phase transitions. Distillation of a solid will occur at pressures and temperatures above the triple point, represented by the green line in the phase diagram. The blue line represents the temperature and pressure conditions where sublimation occurs.
Principles
Many inorganic compounds are solids and therefore it is important to understand the methods for purification of solids. Some of the techniques for the purification of solids are similar to those used for the purification of liquids. For example, distillation is a useful purification technique for low-melting solids, which melt before they vaporize. On a phase diagram, note that distillation can be accomplished at pressures that are above the triple point of a compound (Figure 1). After initially melting to yield a liquid (red line, Figure 1), distillation proceeds as it would for any other liquid-phase compound.
Sublimation is related to distillation, but does not involve the intermediate phase transition to the liquid phase. Sublimation only occurs at specific temperatures and pressures that lie below the triple point of a compound in its phase diagram (Figure 1). Sublimation is a purification technique where a solid is heated (sometimes under vacuum) resulting in a phase transition directly from the solid-phase to the gas-phase. Deposition of the vaporized compound on a cold surface results in isolation of the sublimed material. Non-volatile impurities are left behind after the sublimation is complete. Common examples of substances that readily undergo sublimation at atmospheric pressure are ice (at temperatures below 0 °C) and CO2.
Depending on the volatility of the solid being sublimed, various apparatuses can be used. For highly volatile solids (compounds with a triple point at a high pressure and low temperature), it is possible to make a simple sublimation chamber using a beaker and watch glass. Such an apparatus is appropriate for compounds that sublime at or near atmospheric pressure and ambient temperature. If vacuum and/or inert gas are needed, glassware specifically designed for sublimation (i.e., a sublimation chamber) can be used. The sublimation chamber (Figure 2) allows for sublimation under vacuum or under an inert atmosphere. It is comprised of two glass pieces: the solid is put at the bottom of the main chamber and upon sublimation the purified material is collected on the long cylinder in the center of the chamber called a cold finger, which can be filled with ice-water, dry ice and acetone, or some other cryogen. The base and the cold finger are sealed with an O-ring and secured with a clamp. Upon completion of the sublimation, the chamber can be dissembled (in the air for non-air sensitive compounds or in the glovebox for air-sensitive materials) and the purified solid can be scraped off of the cold finger. All non-volatile impurities should remain at the bottom of the sublimation chamber.

Figure 2. A sublimation chamber designed for low pressure sublimation.
Subscription Required. Please recommend JoVE to your librarian.
Procedure
1. Setup of the Schlenk Line
For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" and "Degassing Liquids" videos in the Essentials of Organic Chemistry series. Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condenses and is explosive in the presence of organic solvents. If it is suspected that O2 has been condensed, or a blue liquid is observed in the cold trap, leave the trap cold under dynamic vacuum. Do NOT remove the liquid N2 trap or turn off the vacuum pump. Over time the liquid O2 will sublime into the pump - it is only safe to remove the liquid N2 trap once all of the O2 has sublimed.
- Close the pressure release valve.
- Turn on the N2 gas and the vacuum pump.
- As the Schlenk line vacuum equilibrates, prepare the cold trap with either liquid N2 or dry ice/acetone.
- Assemble the cold trap.
2. Add 500 mg (2.7 mmol) of ferrocene to the base of the sublimation chamber.
3. Assembly of the Sublimation Chamber
- Place the O-ring in the groove of the chamber base.
- Gently place the cold finger into the chamber base and make sure that the O-ring fits into the groove of the glassware.
- Secure the two pieces of the sublimation chamber using a clamp.
4. Connect the sublimation chamber to the Schlenk line and open the chamber to vacuum for 1 min. Close the vacuum valve on the sublimation chamber. The sublimation will be carried out under static vacuum.
5. Fill the cold finger with an ice bath.
6. Place the base of the sublimation chamber into a water bath heated to 80°C.
7. After the sublimation is complete, remove the sublimation chamber from the bath.
8. Close the stopcock on the Schlenk line.
9. Remove the Schlenk line tube from the sublimation chamber and repressurize the sublimation chamber by slowly opening the valve. Be careful! If the chamber is repressurized too quickly it will disturb the purified crystals on the cold finger.
10. Unclamp the sublimation chamber and remove the water from the cold finger with a pipette.
11. Carefully lift the cold finger out of the sublimation chamber.
12. Scrape the purified ferrocene from the cold finger and transfer to a vial. Record the weight of the purified product. If the compound being sublimed is air-sensitive, the entire apparatus should be brought into an inert-atmosphere glovebox prior to opening the sublimation chamber.
Sublimation is the phase transition of a substance from solid into gas without passing through its intermediate liquid phase. It is an important technique used for purification of organic and inorganic solids.
Usually the transition from solid to gaseous state requires passing through its liquid state.
However, reduced pressure and heating of a solid can lead to volatilization without melting, known as sublimation. The reverse process in which the substance passes from its gaseous to its solid state, is called deposition.
This video will illustrate the principles of sublimation, a typical procedure, and several applications.
At normal pressures, most chemical compounds and elements possess three different states of matter at different temperatures with a triple point at which all three states are present.
As seen in a phase diagram, vaporization and condensation - together known as distillation - may be performed at pressures above the compound's triple point.
On the contrary, sublimation and deposition occur only at pressures that lie below the triple point.
Sublimation can be performed using two types of apparatus, depending on the volatility of the solid: for highly volatile compounds, a makeshift sublimation chamber may be assembled from a beaker and a watch glass. This method is appropriate for compounds that sublime at or near atmospheric pressure and ambient temperature.
If vacuum and/or inert atmosphere are required, a specialized piece of glassware made specifically for sublimation is used. It is made of a glass cup, containing the crude solid, and a hollow cylinder, which contains a cryogen and fits over the top of the cup. An O-ring seals the base and cold finger, and a vacuum attachment makes up the rest of the apparatus.
After completing the sublimation procedure, the apparatus is disassembled in a fume hood or glovebox depending on whether the material is air-sensitive. Then the purified solid may be scraped off of the cylinder, while the non-volatile impurities remain in the cup.
Now that we have discussed the principles of sublimation, let's take a look at an actual procedure.
In a fume hood equipped with a Schlenk line, or dual manifold, weigh 500 mg of ferrocene in the base of a sublimation chamber.
Place an O-ring in the groove of the chamber base, and gently place the cold finger into the chamber base, making sure the O-ring fits. Then secure the two pieces of the chamber with a clamp.
Connect the assembled chamber to the Schlenk line, and open the chamber to vacuum for 1 min. Then close the vacuum valve on the chamber to continue the experiment under static vacuum.
Clamp the chamber to a ring stand and place the base portion of the chamber in an 80 °C bath. Fill the cold finger with an ice-slurry, replenishing it as it warms.
After sublimation is complete, remove the chamber from the bath. Close the stopcock to the Schlenk line and detach the tube from the chamber.
Then, repressurize the chamber by slowly opening the valve to air in a fume hood or glovebox.
Use a pipette to remove water from the cold finger and unclamp the two pieces of the chamber. Then carefully lift the cold finger out of the sublimation chamber.
Scrape the purified ferrocene from the cold finger with a spatula, transfer to a pre-weighed vial, and record the weight.
500 mg of purchased ferrocene was purified via sublimation resulting in 493 mg isolated product with a yield of 99.6%. The proton NMR shows a singlet at 4.17 ppm, which integrates to 10 protons of the ferrocene. The absence of other peaks indicates that no impurities are present, and that the purification was successful.
Now that we have discussed a procedure for sublimation, let's take a look at a few applications.
Water can be sublimed using a process called lyophilization, also known as freeze drying. This is accomplished by freezing a water-filled flask in a dry ice acetone bath at -78 °C and then applying high vacuum by attachment to a lyophilizer, where the water is recaptured in a cold finger.
Many mothballs contain a compound known as naphthalene, which is a simple polyaromatic hydrocarbon, consisting of two fused benzene rings.
Naphthalene sublimes at atmospheric pressure and 80 °C and the gaseous form of this compound is toxic to moths.
You've just watched JoVE's introduction to Sublimation of Ferrocene. You should now understand the principles of sublimation, how to perform an experiment, and several of its applications. Thanks for watching!
Subscription Required. Please recommend JoVE to your librarian.
Results
Ferrocene (99%) was purchased from Alfa Aesar. Sublimation of 500 mg as described resulted in 493 mg isolated product. The purified ferrocene was analyzed by 1H NMR. 1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.17 (s).
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
Sublimation is a technique used in the purification of solids. Solids that sublime at low pressure and temperature are good candidates for purification by sublimation. Here, we have demonstrated how to use a sublimation chamber to sublime ferrocene under static vacuum at 80 °C.
In a laboratory setting, sublimation is a useful technique that can be applied to the purification of solids in a variety of situations including in the purification of starting materials or synthesized products. In this example, the purified solid is collected on the cold finger, while the impurities are left at the bottom of the sublimation chamber. However, one may want to remove a solid impurity that can be sublimed from a non-volatile solid. In this case, the desired material remains at the bottom of the sublimation chamber.
Sublimation is also used in freeze-drying, called lyophilization. Lyophilization is a process to dry materials used in both pharmaceutical and food industries, as well as in research laboratories. In the lyophilization process, a material is first frozen, followed by reduction of the surrounding pressure, which allows the water (or other solvent) to be removed by sublimation.
Subscription Required. Please recommend JoVE to your librarian.
References
- Kaplan, L., Kester, W. L., Katz, J. J. Some properties of iron biscyclopentadienyl. J Am Chem Soc. 74, 5531-5532 (1952).




