As we move into a new year of exciting science, grant proposals, teaching and publications, I find myself pondering over how research in 2016 has advanced our understanding, specifically in the field of Developmental Biology.
Developmental biology is a classical field of research that allows us to understand and make strides toward answering the quintessential question of ‘how living organisms develop from tiny single cells’. The sheer amount of groundbreaking research that was done this year makes it hard to highlight all the many different ways in which the field of Dev. Bio. has advanced recently. However, with 2016 coming to a close and “year in review” articles getting published everywhere, I thought it would be a shame not to highlight at least a few of the Dev. Bio. developments that have just come to light. To that end, here are some excellent examples of the discoveries that have furthered our knowledge in this field.
Human embryos can be grown outside the uterus for 13 days
(Shahbazi et al, 2016; Deglincerti et al, 2016)
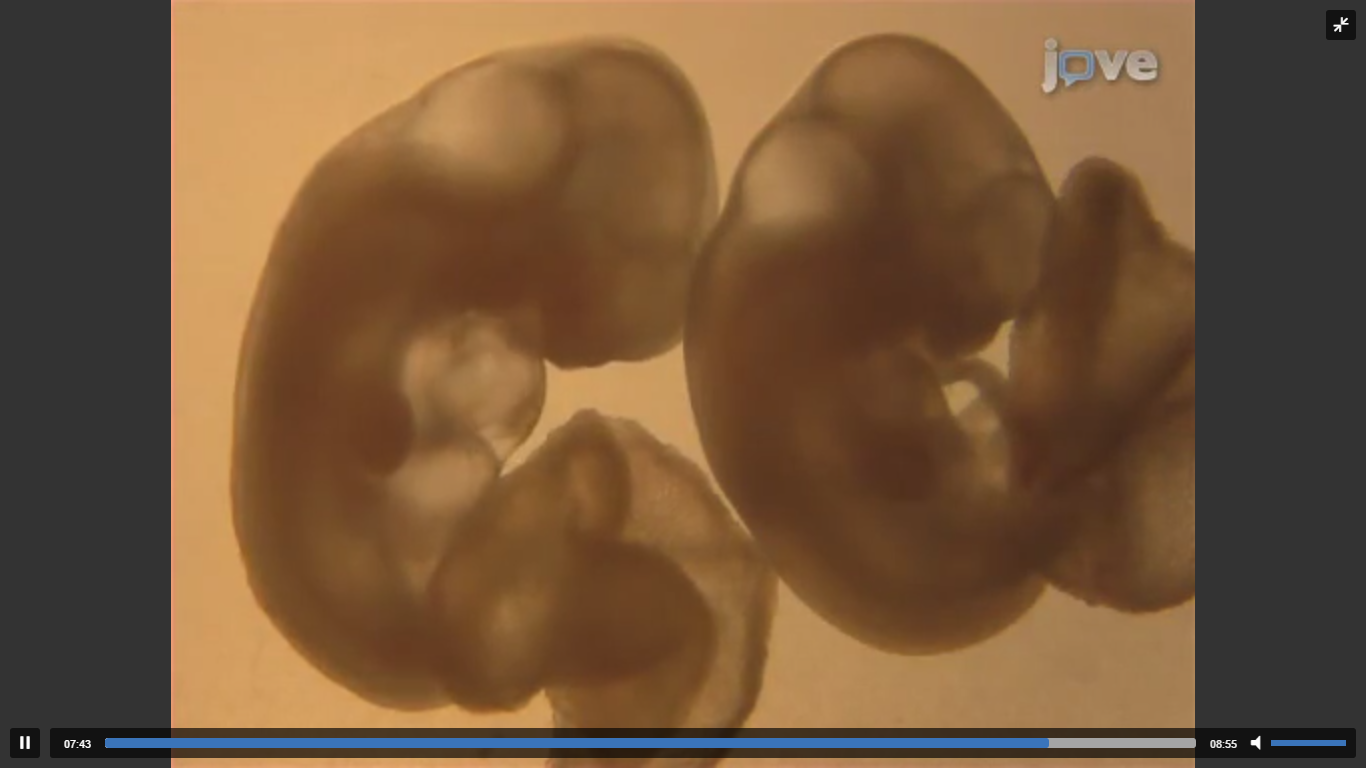
Two research teams in separate studies reported that human embryos could now be grown in-vitro for up to 13 days. This groundbreaking discovery not only advances infertility treatment and research but also allows us to study and understand some of the important biological decisions that are made during early development in humans. Some surprising discoveries have already been highlighted on the basis on this novel access to developmental cues that this system provides. One of the biggest surprises was that the embryo was able to set in motion development of key organs without any biological cues from the mother. These insights may just force us to re-think the way in which early development has been perceived so far. Above is a JoVE article which also highlights the study of early embryonic development in-vitro, though in mice.
3-D lung in a dish from stem cells
(Wilkinson et al, 2016)

Stem cells derived from adult human lungs were used to coat hydrogel beads which were then partitioned into small wells. In each well, the lung cells grew around the beads and formed a three dimensional pattern. This simple and elegant technique led to the development of lung organoids which have important applications in the study of lung diseases and treatment. The study of some lung diseases such as idiopathic pulmonary fibrosis has been difficult when using the conventional methods available so far. However, this advance in lung organoid research greatly enhances the ease with which researchers can study the biological underpinnings of idiopathic pulmonary fibrosis and other lung diseases. Above is a JoVE article which also highlights the importance of organoid culture systems in study of associated diseases and their treatment.
Spinal cord injury side effects in mice treated with human stem cells
(Fandel et al, 2016)
Spinal cord injury patients suffer from a variety of side effects in addition to paralysis. Some of these side effects such as neuropathic pain and bladder dysfunction are caused due to overactive spinal cord circuits. Researchers at UCSF used GABA (gamma-Aminobutyric acid), an inhibitory neurotransmitter, to address this problem. MGE-calls which are GABAergic inhibitory neuron precursors were derived from human embryonic stem cells and placed into the spinal cords of mice after two weeks of induced injury. This inventive approach greatly alleviated some of the side effects associated with spinal cord injury in these animals. This approach holds immense promise for the treatment of spinal cord injury side effects in patients. Above is a related JoVE article which highlights transplantation of neural stem cells in a mouse model of spinal cord injury.