Summary
This article provides an efficient and feasible method for constructing multilayered stem cell sheets with favorable stem cell property.
Abstract
Stem cell therapy shows a promising future in regenerating injured organ and tissues, and the cell sheet technique has been developed to improve the low cell retention and poor survival within the target zone. However, during the in vitro construction process, a solution for maintaining stem cell bioactivity and increasing the cell amount within the cell sheet is urgently needed. Here, this protocol presents a method for constructing a multilayered cell sheet with favorable stem cell bioactivity and optimal operability. Decellularized porcine pericardium (DPP) is prepared by phospholipase A2 (PLA2) decellularization method as the cell sheet scaffold, and rat bone marrow mesenchymal stem cells (BMSCs) are isolated and expanded as the seeded cells. The temporary multilayered cell sheet structure is constructed by using RAD16-I peptide hydrogel. Finally, the cell sheet is cultured with a dynamic perfusion system to stabilize the three-dimensional (3D) structure, and the cell sheet could be obtained following a 48-hour culture in vitro. This protocol provides an efficient and feasible method for constructing a multilayered stem cell sheet, and the cell sheet could be developed as a favorable stem cell therapy product in the future.
Introduction
Stem cell therapy has been reported as an effective treatment for many diseases; however, low cell retention and poor survival within the target zone remain critical issues following traditional stem cell injection. To solve this problem, tissue engineering scientists developed the cell sheet technique. A monolayered cell sheet with intact extracellular matrix was firstly prepared by using the temperature-response culture dish1, and its follow-up studies reported the significant improvements of stem cell retention and survival within the infarcted area2,3. Among the methods, constructing the multilayered cell sheet has been reported as an effective strategy for improving the cell survival and the cell sheet therapeutic effect3,4. Since then, scientists have worked on developing different cell sheet construction methods in order to increase the cell amount, stem cell property, and mechanical property of the cell sheets. So far, certain types of cell sheet have been constructed and studied in the treatment of myocardial infarction5, cartilage injury6, and skin wound7.
The bioactivity of stem cells before transplantation showed an emerging influence on injured tissue regeneration, and different cell sheet construction strategies have different effects on the stem cells. On one hand, confluent cell sheets only consisted of high-density stem cells, and natural extracellular matrices could be acquired by stacking monolayered cell sheets8 or by using magnetic tissue engineering techniques9. On the other hand, researchers developed different scaffolds to provide adequate mechanical strength and support cell growth10,11,12, which allowed a low stem cell seeding density to ensure the nutrition supply. However, despite these approaches, the low efficient nutrition supply within the multilayered cell sheet structure remains a major concern during the in vitro construction. Therefore, an efficient and feasible cell sheet construction system is urgently required.
This protocol describes the steps to prepare a multilayeredmesenchymal stem cell (MSC) cell sheet. In this construction system, the cell sheet mechanical strength is provided by a DPP. Based on this scaffold, the 3D cell structure can be quickly constructed with RAD16-I peptide hydrogel, and a dynamic perfusion system is used to culture the multilayered cell sheet, in order to stabilize the 3D cell sheet structure and provide sufficient nutrition supply for the cells. Using this system, a multilayered BMSC sheet was successfully prepared and exhibited an optimal therapeutic effect on the rat myocardial infarction model13.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
All stem cell and animal experiment procedures were conducted according to the ethical guidelines of the National Guide for the Care and Use of Laboratory Animals and approved by the Jinan University Animal Care and Use Committee (Guangzhou, China).
1. Preparation of the DPP Scaffold with the PLA2 Decellularization Method14
Note: See Figure 1A for a schematic of the PLA2 decellularization method.
- Prepare 100 mL of 200 U/mL PLA2 solution. Add 0.5 g of sodium deoxycholate and 2 mL of PLA2 in 198 mL of carbonate buffer solution.This solution should be used within 24 h after its preparation.
- Obtain the fresh porcine pericardium (FPP) from the slaughterhouse and return to the laboratory within 1 h.
NOTE: The FPP should be stored at 4 °C during the transportation. Steps 1.2 - 1.10 should be conducted with continuous shaking in a thermostat-controlled water bath. - Thoroughly wash the FPP with 200 mL of phosphate buffer solution (PBS) containing 1% penicillin-streptomycin in a 500 mL beaker at 10 °Cfor 10 min. Repeat this step 2x.
- Split the FPP into two layers and remove the adipose tissue with forceps and scissors.
NOTE: Keep the FPP wet by adding 50 mL of PBS every 20 min during the removal of adipose tissue. - Shape the FPP into 10 x 10 cm2 pieces with scissors. Wash the FPP with 200 mL of carbonate buffer solution (CBS) containing 1% penicillin-streptomycin in a 500 mL beaker at 10 °C for 10 min. Repeat this step 2x.
- Transfer the FPP to pure water and soak it at 10°C for 12 h.
- Soak the 10 x 10 cm2 samples in 50 mL of CBS containing 200 U/mL PLA2 and 0.5% (w/v) sodium deoxycholate solution at 37 °C for 6 h.
- Wash the samples with CBS containing 1% penicillin-streptomycin at 10 °C for 10 min. Repeat this step 2x.
- Soak each sample in 50 mL of CBS containing 200 U/mL PLA2 and 0.5% (w/v) sodium deoxycholate solution at 37 °C for 2 h.
- Wash the samples with CBS containing 1% penicillin-streptomycin at 10 °C for 2 h. Repeat this step for 10x at least. Place the samples on flat plates and dry them to constant weight in a constant temperature oven at 55 °C.
NOTE: The sample needs to be completely dried. Weigh the DPP sample every 10 min and repeat that 3x or until the weight no longer changes. - Shape each DPP sample into a 10.5 mm-diameter circle with a trephine. Pack each DPP in a sterile sealed bag.
- Sterilize the DPP samples by gamma irradiation (25 kGy). Stored the DPP samples at 4 °C before use.
NOTE: All samples can be stored for up to six months at 4 °C.
2. Preparations for the Cell Sheet Construction
- Autoclave all instruments and tissue carrier components at 121 °C for 30 min, including 1.5 mL centrifugal tubes, forceps, toothed forceps, scissors, black bases (tissue carrier component), and white tension rings (tissue carrier component).
- Prepare 20 mL of germ-free 10% sucrose solution. Weight 2 g of sucrose and dissolve the sucrose in 18 mL of ultrapure water. Autoclave the 10% sucrose solution at 121 °C for 30 min or filter the solution with a 0.22 µm filter.
- Autoclave the dynamic perfusion system devices at 121 °C for 30 min, including a gas exchange equipment, a 500 mL glass bottle, a perfusion culture container, and the connective tubes.
- Prepare the autoclaved instruments and tissue carrier components.Put the black base part of the tissue carrier in a culture dish.
- Pick up a dried DPP scaffold and put it in the center of the black base.Put a white tension ring on the DPP scaffold and fix it in the tissue carrier.
NOTE: Ensure the scaffold is totally fixed in the tissue carrier and there is no gap between the black base and the white tension ring. If not, separate the tissue carrier and fix the scaffold again. - Add 100 µL of culture medium on the DPP scaffold for rehydration.
NOTE: If the scaffold is not fixed well in the tissue carrier, the culture medium will infiltrate the culture dish. - Put the scaffold into a 37 °C incubator and allow it to soak for 15 min.
3. Preparation of the Cells for Cell Sheet Construction
Note: This protocol is for cell culture using a 100 mm dish. See Figure 1B for a schematic of the construction of the multilayered cell structure.
- Isolate BMSCs13.
NOTE: This method is designed for constructing a multilayered MSC cell sheet. Rat BMSCs are used in this protocol. The BMSCs are isolated using the whole bone marrow adherent method, and the BMSCs are expanded in vitro to obtain enough cell amount.- Autoclave the instruments at 121 °C for 30 min, including forceps, toothed forceps, and scissors. Prepare a 2 mL injection syringe and BMSC culture medium (Dulbecco's modified Eagle's medium [DMEM], 10% fetal bovine serum, 1% glutamine, and 1% penicillin-streptomycin).
- Euthanize the three-week-old male Sprague-Dawley (SD) rats by cervical vertebra dislocation. Soak the animal in 100 mL of 75% alcohol solution in a beaker for 5 min.
- Take the animal out of the beaker and place it prone on the operation table. Incise the skin on the back of the animal with scissors and forceps. Isolate the skin and muscle tissues to expose the thigh femurs.
- Isolate the thigh femurs and put it in 30 mL of PBS in a 50 mL centrifuge tube. Place two thigh femurs into one tube. Vortex the centrifuge tube to wash the tissue thoroughly. Repeat this step 2x.
- Cut both ends of the femurs with scissors and expose the marrow cavity.
- Aspirate 2 mL of BMSC culture medium with an injection syringe. Insert the needle into the marrow cavity and flush out the bone marrow with culture medium. Flush out every two thigh femurs into one 100 mm culture dish.
- For each 100 mm culture dish, add 2 mL of culture medium in the culture dish. Put the culture dish into the 37 °C incubator and static culture for 72 h.
- Take out the culture dish from the incubator. Replace the supernatant with 6 mL of fresh culture medium.
- Observe primary BMSCs under a microscope. Following this, passage the BMSCs every 5 - 7 d.
- Take the cells out of the incubator. Observe the cells under a microscope and choose suitable cells for cell sheet construction. When the BMSCs reach 80% - 90% confluence, the cells can be chosen as the seeded cells.
- Remove the culture medium from the culture dish. Gently wash the cells with 2 mL of warm PBS. Remove all PBS from the culture dish and make sure no liquid remains. Add 2 mL of 0.25% trypsin (or another dissociating solution) to the dish and incubate at 37 °C for 3 min.
- Stop the trypsin effect by adding 2 mL of the culture medium, and gently wash the cells from the dish. Transfer the cell suspension into a new 15 mL centrifuge tube. Centrifugate the cells at 225 x g for 5 min.
- Remove the supernatant. Resuspend the cells with 3 mL of 10% (w/v) sucrose solution.
NOTE: 10% (w/v) sucrose solution is used to wash the cells in order to obtain a uniform cell-hydrogel mixture in the following steps. - Aspirate 10 µL of the cell suspension and count the cell number with a hemocytometer. Calculate the volume needed for the next step. For one cell sheet, three million BMSCs are used.
- Extract three million cells and transfer them into a new 15 mL centrifuge tube. Centrifuge the cells at 225 x g for 5 min.
- Remove the supernatant. Resuspend the cells with 1 mL of 10% (w/v) sucrose solution. Transfer the cell suspension into a 1.5 mL centrifuge tube.
NOTE: Using a 1.5 mL centrifugal tube is beneficial for preparing the cell-hydrogel mixture. - Centrifuge the cells at 260 xg for 5 min. Completely remove the supernatant and obtain the cell sediment.
4. Preparation of the BMSCs and the RAD16-I Peptide Hydrogel Mixture
Note: See Figure 1B for a schematic of the construction of the multilayered cell structure.
- Add 20 µL of 10% (w/v) sucrose solution to the 1.5 mL centrifugal tube. Gently resuspend the BMSCs and obtain a uniform suspension.
NOTE: Do not generate any bubbles during the resuspension. - Add 20 µL of RAD16-I peptide hydrogel at the top of the suspension. Gently stir the RAD16-I peptide and cell suspension with the pipette tip. When the cell suspension and hydrogel are mixed together, gently pipette the mixture a couple of times.
- Take out the DPP scaffold from the tissue carrier and gently aspirate the culture medium with a pipette tip.
NOTE: Ensure the DPP scaffold is fully rehydrated before adding the cell-hydrogel mixture. - Aspirate the mixture and evenly add it to the DPP scaffold.
NOTE: The total volume of the mixture would be about 40 - 50 µL. It is recommended to add the mixture 10 µL at a time from the center to the outside of the scaffold. - Add 1 mL of culture medium to the bottom of the tissue carrier. Put the cell sheet in the 37 °C incubator for 5 min.
- Take out the cell sheet from the incubator. Gently add 4 mL of culture medium in the culture dish and immerse the cell sheet. Put the cell sheet in the 37 °C incubator for 2 h of static culture.
5. In Vitro Culture of a 3D Multilayered Cell Sheet Using a Dynamic Culture System
NOTE: See Figure 1C for a schematic of the 3D dynamic system.
- Prepare the dynamic perfusion system, including a peristaltic pump, gas exchange equipment, a 500 mL glass bottle, a perfusion culture container, and the connective tubes. Assemble the dynamic perfusion system as shown in Figure 2.
- Add 200 mL of culture medium to the sterile glass bottle. Insert the cell sheet into the chamber of the culture container.
NOTE: Pay attention to the direction of the upper surface of the cell sheet. - Add 3 mL of culture medium in the tissue container and close the container. Put the dynamic perfusion system in the incubator and start the pump. Set the flow rate of the peristaltic pump at 8 mL/min. Culture the cell sheet in the dynamic perfusion system for 48 h.
6. Obtaining the Multilayered MSC Cell Sheet
- Autoclave the instruments and tissue carrier components at 121 °C for 30 min, including 1.5 mL centrifugal tubes, forceps, and toothed forceps.
- Pull out the input duct from the glass bottle to stop the supply of culture medium to the container.
NOTE: Stop the peristaltic pump when the culture container is empty. - Take out the cell sheet from the culture container and put it in a culture dish.
- Use one forceps to immobilize the tissue carrier and use another toothed forceps to separate the white tension ring from the black base. Finally, obtain the multilayered BMSCs cell sheet.
- For short preservation, each cell sheet can be transferred to a 1.5 mL centrifugal tube with the forceps. The DPP scaffold should be attached to the inner wall of the centrifugal tube, and the cell sheet should spread out as much as possible in the centrifuge tube.
- Gently add 1 mL of culture medium in the centrifugal tube to immerse the cell sheet. Close the cap of the centrifugal tube and store the cell sheet at 4 °C.
NOTE: The cell sheet should be transplanted or analyzed as soon as possible. It is recommended to use the cell sheet within 4 h.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
The schematic of the multilayered stem cell sheet construction is shown in Figure 1. Preparing the cell sheet scaffold by the PLA2 decellularization method is the first step. Based on the scaffold, a temporary 3D cell structure is constructed by mixing the stem cells with the RAD16-1 peptide hydrogel. In order to obtain a multilayered cell sheet with favorable stem cell bioactivity and optimal mechanical strength, the cell sheet is cultured in a dynamic perfusion system. Under the dynamic nutrition supply, the stem cells are allowed to proliferate and establish cell contacts within the multilayered cell sheet, and the final stable multilayered cell sheet product can be obtained after a ~24- to 72-hour cultivation.
In this case, the cell sheet scaffold DPP is prepared by the PLA2 decellularization method. The appearance of dried DPP is flat, smooth, and semitransparent (Figure 3A). Owing to the specific lyse effect of PLA2, the heterogeneous cells can be completely removed while the ultrastructure of the natural collagen within the DPP scaffold is well-preserved (Figure 3B), and this is important for maintaining the mechanical strength and biocompatibility of the scaffold. Additionally, the scaffolds can be modified as a growth factor control release system to support stem cell growth and improve the in vivo regeneration13.
When the stem cells reach ~80% - 90% confluence, the cells are isolated from the culture dish and washed with a 10% sucrose solution. After centrifugation, the cells are mixed with the RAD16-I peptide hydrogel and added to the rehydrated DPP scaffold. A temporary multilayered structure is formed following a two-hour static culture. Finally, the multilayered BMSC sheet product (Figure 4) is acquired following a 48-hour culture in the dynamic perfusion system. With the support of the DPP scaffold, the cell sheet can be easily manipulated with forceps, and it can be temporarily preserved in culture medium in the 1.5 mL tube at 4 °C for 4 hours before examination or transplantation (Figure 4). As the immunofluorescence staining result shows, the BMSCs are highly positive for the stem cell markers CD90 and CD29. After the cell sheet construction, the BMSCs within the multilayered cell sheet show high levels of CD29 and CD90 (Figure 5).

Figure 1: The flowchart of constructing the multilayered stem cell sheet. (A) By using the PLA2 decellularized method, the heterogeneous cells within the FPP are destroyed while the natural extracellular matrices are well-preserved in the DPP scaffold. (B) Based on the DPP scaffold, the temporary multilayered cell structure is constructed by mixing the stem cells and self-assembling peptide hydrogel. (C) To follow, the cell sheet is cultured in a 3D dynamic system, and the stem cells are expected to proliferate and establish cell contacts under the dynamic nutrition supply. Please click here to view a larger version of this figure.

Figure 2: The tissue carrier and the dynamic perfusion system. (A) This panel shows the 13 mm-diameter tissue carrier. (B) This panel shows the assembly of the dynamic perfusion system. Please click here to view a larger version of this figure.
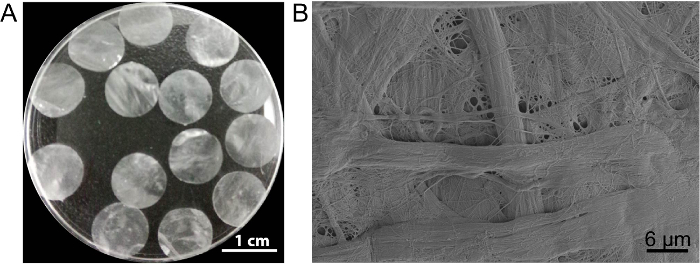
Figure 3: The appearance and ultrastructure of DPP. (A) This panel shows the appearance of the 10.5 mm-diameter DPP scaffolds. (B) This panel shows a representative image of the scanning electron microscope (SEM) result of the DPP scaffold. Please click here to view a larger version of this figure.
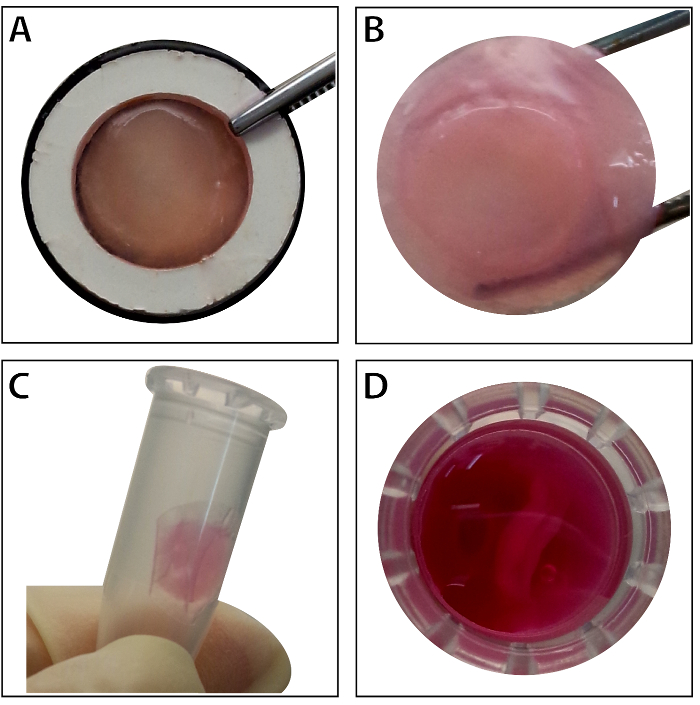
Figure 4: The appearance of the multilayered BMSC sheet. (A) This panel shows the appearance of the multilayered BMSC sheet within the tissue carrier. (B) The intact multilayered BMSC sheet is held by forceps. (C - D) The multilayered cell sheet can be preserved temporarily in the 1.5 mL tube before use. Please click here to view a larger version of this figure.
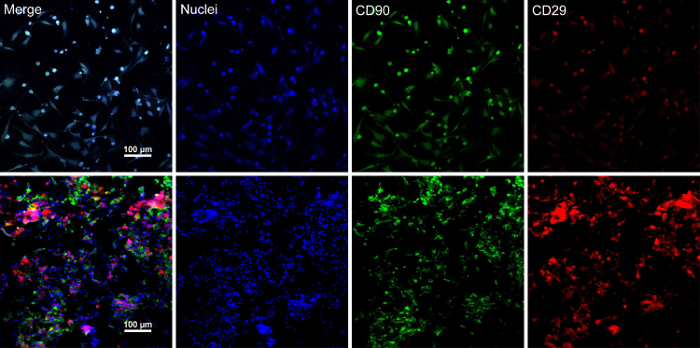
Figure 5: Immunofluorescence staining results of BMSC markers expression. (A) This panel shows immunofluorescence staining results of BMSCs before cell sheet construction. (B) This panel shows immunofluorescence staining results of the multilayered BMSC sheet section. CD90 (green) and CD29 (red) were positively expressed in the BMSCs and the cell sheet. Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
The present protocol reports an efficient method for constructing a multilayered MSC sheet. This cell sheet exhibits optimal mechanical strength, high cell seeding density, and favorable stem cell bioactivity. Using BMSCs as an example, the 3D cell structure is quickly constructed with RAD16-I peptide hydrogel. After being cultured in the dynamic perfusion system, the multilayered BMSC sheet is successfully obtained and the BMSCs maintain a high expression of stem cell markers.
Constructing the temporary multilayered cell structure is the critical step of the protocol. The RAD16-I is a commercial hydrogel peptide, and it consists of 1% amino acid and 99% water. Several studies reported that this peptide hydrogel can mimic the natural ECM environment and is beneficial for stem cell proliferation and survival15,16,17. In the present protocol, a three million MSC suspension (in 20 µL of 10% sucrose solution) was mixed with 20 µL of RAD16-I peptide hydrogel. The volume ratio of the cell suspension and the peptide hydrogel was 1:1. This peptide hydrogel is sensitive to the environmental pH value, and the peptide molecules would automatically form the 3D network when the pH value changes from acid to neutral. Because the cell surface contains charged particles, the cell mixture changed from liquid to hydrogel in a short time, which has influences the even mixing of the cells. A favorable cell-hydrogel should be an even mixture of the cell suspension and the peptide hydrogel and enables the cell mixture to be evenly added onto the scaffold. The researchers can optimize the mixture condition by altering the seeded cell number, sucrose solution volume, and the peptide hydrogel volume according to their actual need. It is worthwhile to notice that washing the cells with 10% sucrose solution and evenly mixing the cell-hydrogel mixture are the critical steps of the protocol, and an uneven mixture could cause great cell loss and an unstable temporary multilayered structure.
After adding the cell-hydrogel mixture onto the DPP scaffold, the mechanical strength of the multilayered cell sheet structure is weak because the peptide hydrogel network is not strong enough to maintain the long-term multilayered cell structure, and cell connections and ECM secretions are needed to enhance the stability of the cell sheet. Moreover, the dynamic infiltration of the culture medium can facilitate the stem cells to proliferate and establish cell contacts within the multilayered cell structure, while an insufficient nutrition supply will cause cell apoptosis and reduce the cell density of the cell sheet13. Therefore, the dynamic perfusion system is important for stabilizing the multilayered cell sheet structure. In addition, the appropriate flow rate of the culture medium should be adjusted according to the specific stem cell type and cell seeding density. Also, the weak mechanical connection between the DPP scaffold and the multilayered cell structure remains the limitation of the present construction method, which may cause the division of the multilayered cell layers and the scaffold. Therefore, further studies are required to enhance the mechanical biocompatibility of the 3D hydrogel scaffold and the DPP scaffold.
So far, tissue engineering scientists have been focusing on establishing efficient nutrition supply systems in vitro, such as coculturing endothelial cells18 and using a porous scaffold19. However, the nutrition permeability within the 3D structure is low in the traditional static 3D culture system, and the stem cell viability will be greatly affected. In this case, using the dynamic perfusion system can provide enough nutrition supply to maintain stem cell viability. Using this protocol, a multilayered BMSC sheet improved the cardiac function and angiogenesis in a rat myocardial infarction model13. Constructing a stem cell sheet product with a high cell load and favorable stem cell property is significant to the tissue regeneration. Using this efficient constructed method, different kinds of multilayered stem cell sheets could be constructed by altering the seeded stem cell types, such as epithelial stem cell sheet, neural stem cell sheet, or cardiac stem cell sheet. Further explorations of and alternatives to the multilayered stem cell sheet are expected to expand the applications for more tissue regeneration.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 31771064); the Science and Technology Planning Project of Guangdong Province (grant numbers 2013B010404030, 2014A010105029, and 2016A020214012); the Science and Technology Planning Project of Guangzhou (grant number 201607010063); and the Undergraduate Innovation and Entrepreneurship Training Program (grant number 201610559028); the National Science Foundation for Young Scientists of China (grant number 31800819).
Materials
| Name | Company | Catalog Number | Comments |
| Phospholipase A2 | Sigma-Aldrich | P6534 | |
| Sodium deoxycholate | Sigma-Aldrich | D6750-100G | |
| Phosphate buffer | Gibco BRL | 89033 | |
| Penicillin streptomycin / amphotericin | Gibco BRL | 15640055 | |
| Buffer bicarbonate | Sigma-Aldrich | C3041 | |
| Table concentrator | Changzhou Aohua Instrument Co. | KT20183 | |
| Dulbecco's Modified Eagle Medium(DMEM) | Corning Cellgro | 10-014-CVR | |
| South American fetal bovine serum | Gibco BRL | 10270-106/P30-3302 | |
| L-Glutamine | Corning Cellgro | 25-005-CI | |
| 0.25% Trypsin/2.21 mM EDTA | Corning Cellgro | 25-053-CI | |
| Biosafety cabinet | Esco,Singapore | AC2-2S1 | |
| Constant temperature incubator | Esco,Singapore | CLS-170B-8 | |
| Centrifuge tube | Corning | 430790 | |
| EP tube | Axygen | 31617934 | |
| Centrifugal machine | TOMOS | 1-16R | |
| Sucrose | Sigma-Aldrich | S9378-500G | |
| Pura Matrix | BD | 354250 | |
| Dynamic perfusion culture system | Minucells and Minutissue | D-93077 | |
| Peristaltic pump | Ismatec | IPC N8 | |
| Pump tubing | Ismatec | Nr.1306 | |
| MINUSHEET 1300 | Regensburg | tissue carrier components | |
| MINUSHEET | Regensburg | dynamic perfusion system | |
| MINUSHEET 0006 | Regensburg | gas exchange equipment | |
| MINUSHEET 0002 | Regensburg | 500 mL glass bottle | |
| MINUSHEET 1301 | perfusion culture container |
References
- Miyahara, Y., et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine. 12 (4), 459-465 (2006).
- Narita, T., et al. The use of cell-sheet technique eliminates arrhythmogenicity of skeletal myoblast-based therapy to the heart with enhanced therapeutic effects. International Journal of Cardiology. 168 (1), 261-269 (2013).
- Narita, T., et al. The Use of Scaffold-free Cell Sheet Technique to Refine Mesenchymal Stromal Cell-based Therapy for Heart Failure. Molecular Therapy. 21 (4), 860-867 (2013).
- Matsuo, T., et al. Efficiently Piled-Up Cardiac Tissue-Like Sheets With Pluripotent Stem Cell-Derived Cells Robustly Promotes Cell Engraftment and Ameliorates Cardiac Dysfunction After Myocardial Infarction. Circulation. 128 (22), (2013).
- Alshammary, S., et al. Impact of cardiac stem cell sheet transplantation on myocardial infarction. Surgery Today. 43 (9), 970-976 (2013).
- Chen, G. P., et al. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. Journal of Biomedical Materials Research Part A. 67a (4), 1170-1180 (2003).
- Cerqueira, M. T., et al. Human Adipose Stem Cells Cell Sheet Constructs Impact Epidermal Morphogenesis in Full-Thickness Excisional Wounds. Biomacromolecules. 14 (11), 3997-4008 (2013).
- Sasagawa, T., Shimizu, T., Sekiya, S., Yamato, M., Okano, T. Comparison of angiogenic potential between prevascular and non-prevascular layered adipose-derived stem cell-sheets in early post-transplanted period. Journal of Biomedical Materials Research Part A. 102 (2), 358-365 (2014).
- Ishii, M., et al. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. International Journal of Cardiology. 175 (3), 545-553 (2014).
- Godier-Furnemont, A. F., et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proceedings of the National Academy of Sciences of the United States of America. 108 (19), 7974-7979 (2011).
- Liu, Y., et al. Electrospun nanofibrous sheets of collagen/elastin/polycaprolactone improve cardiac repair after myocardial infarction. American Journal of Translational Research. 8 (4), 1678-1694 (2016).
- Arana, M., et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials. 35 (1), 143-151 (2014).
- Wang, Y., et al. Preparation of high bioactivity multilayered bone-marrow mesenchymal stem cell sheets for myocardial infarction using a 3D-dynamic system. Acta Biomaterialia. 72, 182-195 (2018).
- Wu, Z., et al. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials. 30 (21), 3513-3522 (2009).
- Degano, I. R., et al. The effect of self-assembling peptide nanofiber scaffolds on mouse embryonic fibroblast implantation and proliferation. Biomaterials. 30 (6), 1156-1165 (2009).
- Lampe, K. J., Heilshorn, S. C. Building stem cell niches from the molecule up through engineered peptide materials. Neuroscience Letters. 519 (2), 138-146 (2012).
- Cui, X. J., et al. Transplantation of Mesenchymal Stem Cells with Self-Assembling Polypeptide Scaffolds Is Conducive to Treating Myocardial Infarction in Rats. Tohoku Journal of Experimental Medicine. 222 (4), 281-289 (2010).
- Jun, I., et al. Spatially Assembled Bilayer Cell Sheets of Stem Cells and Endothelial Cells Using Thermosensitive Hydrogels for Therapeutic Angiogenesis. Advanced Healthcare Materials. 6 (9), (2017).
- Chen, C. H., et al. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovascular Research. 80 (1), 88-95 (2008).



