Summary
Presented here is a protocol for a single-cell, epifluorescence microscopy-based technique to quantify grazing rates in aquatic predatory eukaryotes with high precision and taxonomic resolution.
Abstract
Elucidating trophic interactions, such as predation and its effects, is a frequent task for many researchers in ecology. The study of microbial communities has many limitations, and determining a predator, prey, and predatory rates is often difficult. Presented here is an optimized method based on the addition of fluorescently labelled prey as a tracer, which allows for reliable quantitation of the grazing rates in aquatic predatory eukaryotes and estimation of nutrient transfer to higher trophic levels.
Introduction
Heterotrophic prokaryotes are a key biological component in aquatic systems and account for a significant fraction of plankton biomass1,2,3. Factors that control their abundance, diversity, and activity are crucial for understanding their role in biogeochemical cycling (i.e., the fate of organic carbon and other nutrients and flow of energy from prokaryotes to higher trophic levels). Protozoan grazing is one of these important factors. Bacterivory of heterotrophic nanoflagellates and ciliates imposes a strong top-down control over prokaryotic abundance, community function, structure, diversity, and even cellular morphology and growth rate of particular bacterial groups4,5,6. In some systems, protists serve as the major cause of bacterial mortality6,7.
The standard approach used to assess protozoan bacterivory, which has been used for some time now, involves the use of fluorescently labeled bacteria (FLB) as prey analogues and epifluorescence microscopy. Cell-specific uptake rates can be determined by quantifying the number of labeled prey particles in protistan food vacuoles over a selected time course8. There are several advantages to this approach. Tracer is added to natural samples with natural predator and prey assemblages. There is minimum sample manipulation prior to incubation, minimum sample alteration by the added FLB tracer, and incubation times are short to ensure sound results obtained under close to in situ conditions. Alternatively, in environments with low numbers of bacterivorous protists or zooplankton (e.g., offshore marine systems), disappearance rates of FLB added to samples in low amounts (2%-3% tracer) can be detected via flow cytometry in long-term (12-24 h) incubation experiments. Then, numbers of FLB at the start and end points (integrating the impact of all bacterivores) are quantified by flow cytometry (for details, see previous publication9). However, such a parameter only represents total aggregated bacterivory rates that cannot be directly attributed to any particular protistan and zooplankton grazer groups or species.
Overall, quantifying the protistan species- or morphotype-specific bacterial mortality rates in the aquatic environment accurately and with ecological meaning can be challenging. Some protists are selective grazers, and the size and cell shape of the added FLB tracer may distort natural rates of prey ingestion10,11. Moreover, protistan activity and metabolism are highly temperature-sensitive12; therefore, the amount of added FLB tracer needs to be carefully manipulated for each individual sample type (not only based on the natural abundance, size, and morphology of bacteria and prevailing types of bacterivores, but also on temperature). Most studies focus on bulk protistan grazing activity; however, the bacterivory of specific protistan species often holds a much higher information value and may be preferable. In this case, taxonomic knowledge of the protist species present in a sample and understanding of their behavior is needed. Hence, considerable amounts of time and labor are required to obtain sound results on species-specific rates of bacterivory attributable to a particular protistan group or species.
Despite these difficulties, this approach remains the most suitable tool currently available to assess protistan bacterivory in natural settings. Presented here is a comprehensive, easy-to-follow method for using FLB as a tracer in aquatic microbial ecology studies. All of the mentioned problematic aspects of the approach are accounted for and an improved workflow is described, with two experiments from contrasting environments as well as contrasting ciliate species as examples.
The first case study was conducted in an epilimnetic environment from the mesotrophic Římov water reservoir in the Czech Republic, which shows grazer and bacterial abundances comparable to most surface freshwater bodies (cf.5,7). The second case study was conducted in the highly specific environment inside traps of the aquatic carnivorous plant Utricularia reflexa, which hosts extremely high numbers of both grazing mixotrophic ciliates (Tetrahymena utriculariae) and bacterial cells. Calculations of cell-specific grazing rates and bacterial standing stocks in both sample types are shown. A range of ecological interpretations of the results is then discussed, and examples of possible follow-up studies are finally suggested.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
1. Sample collection
- Collection of reservoir water sample: the first case study (Exp I; lower natural in situ predator and prey abundance system)
- Collect water samples from the desired location at a suitable depth. Keep the samples in a temperature-controlled cooler filled at in situ temperature (avoiding temperature shock; it should be noted that uptake rates of protists are temperature-dependent) during transport to the laboratory.
NOTE: Our sampling was conducted in the meso-eutrophic canyon-shaped Římov reservoir (South Bohemia, volume 34.5 x 106 m3, maximal depth 43 m, mean retention time 100 days, dimictic). The sampling site was located at a 30 m depth, close to the dam. A mixed sample at a 0.5 m depth was collected by a 2-l Friedinger sampler. - Continue to section 1.2 as soon as possible.
- Collect water samples from the desired location at a suitable depth. Keep the samples in a temperature-controlled cooler filled at in situ temperature (avoiding temperature shock; it should be noted that uptake rates of protists are temperature-dependent) during transport to the laboratory.
- Collection of fluid from the traps of carnivorous Utricularia reflexa plants: the second case study (Exp II; a system with high predator and prey abundances, extremely small sample volume)
- Gently shake the plants underwater, remove from the cultivation container, and place them on absorbent material to absorb excess water. Depending on the robustness of the plants and size of the traps, choose 8-10 plant individuals.
- Divide each shoot into roughly equal parts by counting leaf nodes bearing trapping organs. Each shoot segment will serve to collect mixed samples representing young, middle-aged, and old traps.
- Attach a thin glass capillary and Eppendorf vial for sample collection to a peristaltic pump. Proceed to insert the capillary tip into the trap opening. Using the vacuum pump, suck out all the fluid from each trap until 900 ± 100 µL of trap fluid is collected for each trap age category.
- Use triplicate subsamples of 200 µL of the pooled trap fluid for protistan grazing experiments. Process these immediately as detailed in section 3. Preserve the remaining ±300 µL of the sample for all other analyses of microbial components living in the fluid, as detailed below (section 2).
- Proceed immediately to section 2.
2. Fixation of collected samples
- Exp I and II: fix water/trap fluid sub-samples for bacterial enumeration (section 4; approximately 20 mL and 0.3 mL, respectively) with formaldehyde for at least 1 h to obtain a 2% final volume:volume concentration in each sample.
NOTE: Handle formaldehyde exclusively in the fume hood, and wear gloves at all times while manipulating samples.
3. Sample filtration
- Sample dilution (Exp I): no dilution is needed for the reservoir water samples. (Exp II): dilute the trap fluid sample 10x-100x with particle-free MQ water to achieve a suitable distribution of the target microbes on filter surfaces prior to counting via epifluorescence microscopy.
- Filter 1-2 mL (Exp I) of the reservoir water or 10-30 μL (Exp II) of the subsample of the trap fluid for bacterial counting onto black 0.2 µm pore-size filters, using a filtration funnel (25 mm diameter).
- Stain the filters with DAPI (4',6-diamidino-2'-phenylindole dihydrochloride, 0.2% final concentration) for 4 min.
NOTE: Avoid skin and working surface contamination, and wear gloves. - Place the filter with concentrated microbes onto a drop of immersion oil (for fluorescent microscopy) on a microscope slide. Place another oil drop onto the center of the filter and cover with a coverslip, making sure that the oil is distributed evenly.
- At this point, preferably process samples immediately, or alternatively, store them in the freezer (-20 °C) for several weeks to months until further analysis.
4. Enumeration of bacterial numbers on the filters
- Place the slide under the epifluorescence microscope (with the filter set corresponding to the fluorochrome DAPI). Place a 10 x 10 counting grid into one of the oculars. Move the slide to a random position.
- Quantify bacterial cells (blue fluorescence) in the area of the counting grid (under the magnification 1000x). In the counts, include the cells crossing the left and upper edges of the counting grid, while excluding those located across the right and lower edges.
- Move to another random position and repeat enumeration on at least 10-15 counting grids, amounting to 500 cells counted in total.
- Establish a conversion factor for a given microscope and magnification, based on knowledge of the ratio of the area of one grid to the total effective filtration area of the filter. Then, divide the total number of cells counted by the number of grids counted, yielding an average number of bacteria per grid.
- Multiply the latter parameter by the conversion factor established and normalize the resulting number per mL of a sample (depending on the volume of sample filtered) to obtain the total bacterial abundance per mL.
5. Determining protistan abundance
- Fix the water sample (Exp I) or trap fluid (Exp II) subsamples with either glutaraldehyde (1% final concentration, more suitable for samples with the presence of chlorophyll containing particles to be processed days to weeks after fixation) or using the formol-thiosulfate decolorization technique specified below.
NOTE: Both preservation techniques prevent egestion of the ingested material from food vacuoles of protists8. - Regarding the formol-thiosulfate decolorization technique, add 100 µL/1 µL of Lugol’s solution into 20 mL/200 µL of the water/trap fluid subsample (Exp I/Exp II, respectively).
- Follow immediately with the addition of 0.5 mL/50 µL of borate-buffered formalin then 20 µL/2 µL of 3% sodium thiosulphate (Exp I/Exp II, respectively).
NOTE: The sodium thiosulphate decolorizes the yellow color of Lugol to enable observation of cells under the epifluorescence microscope8. - Filter a known volume of sample (depending on the number of target protists) onto 1 µm pore-size black polycarbonate filters.
- Estimate the number of the protist species is by counting at least 200 cells under magnification 600x using the counting grid (see above).
- Reduce the volume of the sample under a vacuum in the filtration funnel by low vacuum to approximately 2 mL. Then, release the under-pressure and add the DAPI fluorochrome (4',6-diamidino-2-phenylindole dihydrochloride13, 0.2% final concentration) for 2 min.
6. Determining community structure of ciliates in plankton samples
NOTE: Ciliate communities in freshwater habitats are highly diverse14,15,16,18, and their microscopic determination is challenging. Sorting the ciliate groups into functional guilds10,14,16,17 allows for more detailed analysis of different ciliate groups as pelagic bacterivores.
- Evaluate ciliate community structure by combining the following:
- DAPI-stained samples in epifluorescence microscopy (to localize the ciliate cells with bright fluorescence of macro- and micro-nuclei of different sizes and morphology) combined with the uptake of fluorescently labeled bacteria (FLB8; for details, see below), tracking the ability of ciliates to feed on bacteria.
- Live sample observation in selected cases17,19. For more details of the above approaches and criteria used for grouping ciliates into different taxonomic categories, see previous publications16,17.
NOTE: Cited studies have indicated that among ciliates, omnivorous species from Stichotrichia(genera Halteria and Pelagohalteria) and Oligotrichia (namely Rimostrombidium spp.) are the most important pelagic consumers of bacterioplankton in a vast majority of freshwater habitats10,17,18.
7. Estimating ciliate grazing rates
- Calculate the uptake rates of ciliates on bacteria based on changes in the average number of tracer [i.e., FLB8 per ciliate related to time of incubation (5-15 min)] and tracer amount of FLB added, accounting at most for 5%-15% of total bacteria.
- To compare uptake rates among different protistan species, normalize the uptake rates as the number of bacteria per ciliate per hour, with calculations based on the actual incubation time and proportion of tracer FLB added.
NOTE: The general scheme of the FLB method application to estimate both cell- or species-specific and bulk bacterivory rates in natural samples is depicted in Figure 1. - Preparation of FLB from bacterial strains indigenous to a freshwater environment8
- Select a suitable size (mean cell volume = MCV) and morphology of bacteria so that they effectively mimic the typical sizes of bacterioplankton/bacterial cells in the aquatic system being investigated.
NOTE: For Exp I, a mixture of isolated strains from the study location of the genus Limnohabitans and Polynucleobacter was used (i.e., typical, highly abundant bacterioplankton in lakes and ponds)20. For details on morphology and sizes of the strains, see previous publications3,17,18,21. - Harvest bacterial cells from culture by centrifugation (5,000 x g) for 15 min at the early stationary phase and mix them at a numerical ratio that yields MCV ± SD of the cells in the mixture corresponding to the typical MCV of bacteria at the chosen location.
- Suspend the pellets in 10 mL of phosphate-buffered saline (PBS; pH = 9).
- Add 2 mg of the yellow-green fluorescing dye 5-(4,6-dichlorotriazin-2-yl) aminofluorescein (DTAF, binds to proteins) to the cell suspension in the phosphate-saline buffer, and incubate in a 60 °C water bath for 2 h.
- After the incubation, centrifuge the cells down, decant the DTAF solution, and wash and centrifuge 3x with PBS.
- After the final wash, re-suspend the cells in 20 mL of the PPi-saline buffer.
- Vortex the FLB suspension and pipette 1.5 mL aliquots into 2 mL cryo-vials, then keep frozen (at -20 °C) in PPi-saline buffer until use.
- Pre-filter PPi-saline buffer through a 0.2 µm polycarbonate filter for use in the next step.
- To determine FLB concentration, transfer a small aliquot (usually 20-40 µL) to 2 mL of particle-free PPi-saline buffer, sonicate at 30 W for several 2 s bursts, and filter onto 0.2 µm polycarbonate black filter for enumeration via epifluorescence microscopy (1,000X magnification) under optical filter settings for DTAF (448 nm/520-540 nm).
- Select a suitable size (mean cell volume = MCV) and morphology of bacteria so that they effectively mimic the typical sizes of bacterioplankton/bacterial cells in the aquatic system being investigated.
- Tracer technique for estimation of ciliate bacterivory
- For grazing experiments in natural planktonic habitats, dispense 300 mL samples into well-rinsed 1 L flasks and incubate at in situ temperature for 15 min (to allow protists to recover from the handling shock).
- Add FLB tracers to constitute 5%-15% of total bacteria, with the amounts added depending upon the season and water temperature.
NOTE: There is a very broad, season-dependent spectrum in ciliate species-specific uptake rates spanning several orders of magnitude (i.e., from 101-104 bacteria ciliate-1 per hour)10,16,17,18,22,23,24. - In periods of enhanced occurrence of ciliates with high uptake rates (usually during the summer), also run a parallel incubation with very low FLB additions, constituting only 2%-4% of total bacteria to avoid excessive loading of ciliate vacuoles by tracer FLB (see examples in Figure 2).
- Incubate ciliate/plankton samples with FLB for 5-15 min.
- There are two possibilities for sample fixation to prevent egestion of the ingested material from food vacuoles of protists8. Terminate the incubations by the addition of 1% glutaraldehyde (final concentration that is more suitable for samples with chlorophyll-containing particles, such as algae). Alternatively, use 100 µL/10 µL of Lugol's solution into 20 mL/200 µL of water/trap fluid subsample, followed immediately by the addition of 0.5 mL/10 µL of borate-buffered formalin then 200 µL/2 µL of 3% sodium thiosulphate (Exp I/Exp II, respectively).
- After adding the fixative, let the samples rest for at least 1 h in the dark at 4 °C to ensure thorough preservation of ciliate cells.
- Take natural plankton subsamples from 4-30 mL/10-30 μL (Exp I/Exp II, respectively; the volume depends on ciliate abundance) and stain with DAPI (final concentration 0.2% wt/vol; for details, see step 3.2 above).
- Pass through 1 µm black filters and inspect via epifluorescence microscopy to count ciliates (600x magnification) and enumerate the number of FLB tracers ingested (mostly at 1000x magnification) as detailed in previous publications2,17. Inspect samples within 7 days after preservation.
- To estimate total protozoan/species-specific grazing, multiply average uptake rates of all ciliates, or of only the ciliate species as detected by in situ abundance.
- Example of the calculation of per-cell uptake rates from in situ data from the Římov water reservoir is described as follows:
- Assume that the bacterial concentration is 3.55 x 106 bacteria/mL and tracer FLB added is 0.25 x 106 FLB/mL, which yields a sum of 3.8 x 106 bacteria/mL of total bacterial particles (natural bacteria + FLB = 100% of prey particles) available to phagotrophic protists in the natural sample.
NOTE: The FLB tracers added thus represent 6.58% (a project of 0.25/0.038) of total bacterial particles. The average number of FLB per Halteria sp. is 6.2 FLB in 5 min incubations. - To normalize uptake per hour, use the following calculation: (6.2 x 12)/(6.58/100) = 1131 bacteria per Halteria cell/h.
NOTE: For more examples of distributions of individual uptake rates of Halteria sp. detected under variable water temperatures, amounts (as percents) of tracer FLB added, and different incubation times with FLB, see Figure 3.
- Assume that the bacterial concentration is 3.55 x 106 bacteria/mL and tracer FLB added is 0.25 x 106 FLB/mL, which yields a sum of 3.8 x 106 bacteria/mL of total bacterial particles (natural bacteria + FLB = 100% of prey particles) available to phagotrophic protists in the natural sample.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Example experiment I was run in Římov water reservoir (South Bohemia, CZ), which is a natural site with lower natural in situ predator and prey abundance. Representative data is reported for the omnivorous ciliate species Halteria grandinella, which is an abundant and efficient grazer of picoplankton (<2 µm) particles10,16,17,18,22. Figure 3 shows box-and-whisker plots of numbers of FLB per cell of Halteria sp. from the Římov reservoir (Figure 3A), which was recalculated to rates of bacterial uptake per hour (Figure 3B) detected in four individual experiments conducted in April, May, August, and September. There was high variability in ciliate uptake rates, largely caused by the temporal differences in water temperature.
It should be noted that the Q10 parameter reflects the fact the microbial processes run approximately 2.5x faster with a temperature increase of 10 °C12, which also holds for ciliate uptake rates on bacteria. With this physiological rule in mind, considerably different proportions of FLB and incubation times were used for different seasons (for details, see Figure 3A). Thus, the expected temperature effect was compensated for, and the experimental setting yielded optimized average and median values of uptake rates approximately between 5-10 FLB per ciliate cell. Generally, these amounts of ingested FLB are easily countable (see examples in Figure 2, two left photographs), generating precise estimates of the tracer (mostly being between 1-15 FLB per ciliate) uptake rates. However, due to modified FLB tracer added (%) and different times of sample incubation the absolute values (expressed as number of bacteria grazed ciliate per hour) differed significantly (p < 0.01, Kruskal-Wallis test; followed by Dunn's multiple-comparison test, p < 0.05; see examples in Figure 3B) among the experiments. The data also illustrate typical natural variability in absolute bacterivory rates in the planktonic populations of Halteria grandinella, with a close match of their mean and medium values (Figure 3).
In the presence of highly efficient bacterivorous ciliates in samples, such as peritrichous ciliates, they can become heavily "over-labeled" by FLB in typical tracer amounts of 5%-10% of total bacteria (see right side photograph in Figure 2). This may strongly limit the accurate quantification of ingested FLB. In such cases, it is suggested to run additional parallel incubations with only low amounts of FLB accounting for only 1.5%-3% of total bacteria. However, generally both the tracer amounts as well as incubation times can be manipulated to optimize the number of FLB per cell (Figure 2).
Example experiment II: Displayed is the data from a system with large predator and prey abundances, where only an extremely small sample volume is available to experimentally estimate bacterivory rates of the ciliate Tetrahymena utriculariae25. It is a moderate bacterial grazer living in high abundance exclusively in traps of carnivorous Utricularia reflexa plants26,27. Figure 4 shows box-and-whisker plots of number of FLB per cell of T. utriculariae under different experimental settings (Figure 4A,B) that is recalculated into rates of bacterial uptake per hour (Figure 4C,D) detected in young, mature, and old traps. Interestingly in traps, chloroplast-bearing populations of the ciliate T. utriculariae were detected, while apochloric populations of T. utriculariae were isolated from traps and maintained on mixed bacterial suspension growing on wheat grains in the dark (for details, see Figure 1 in a previous publication26).
The chloroplast-bearing populations live in light-illuminated traps; thus, the chloroplasts can provide an additional organic carbon source and oxygen to the ciliate host. One of the hypotheses tested was that the apochloric ciliate populations grazed bacteria significantly faster, as the bacteria represent the only particulate source of organic carbon available to dark-grown isolated subpopulations of the ciliate.
Indeed, while there were no significant differences in bacterivory rates of the ciliates living in young, mature and old traps of Utricularia reflexa (Figure 4A,C), the apochloric populations of T. utriculariae grazed bacteria significantly (p < 0.01, Kruskal-Wallis test; followed by Dunn's multiple-comparison test, p < 0.05), approximately 3x faster than the chloroplast-bearing ciliates living in young, mature, and old traps (Figure 4C,D). Note that again, both the tracer amounts as well as incubation times (Figure 4A,B, top) were modified to optimize number of FLB per cell (generally between 1-15), with average and median values around 5 FLB/ciliate. These numbers are distinguishable in ciliate food vacuoles and allowed accurate tracer counting. However, expressed in absolute numbers of bacteria grazed per hour, the chloroplast-bearing and apochloric populations grazed approximately 350 and 1,000 bacteria ciliate per hour, respectively. This experimental set-up brought new insights into the metabolic and physiologic traits of two distinct subpopulations of the same ciliate species living under strikingly different environmental constraints25,26,27.
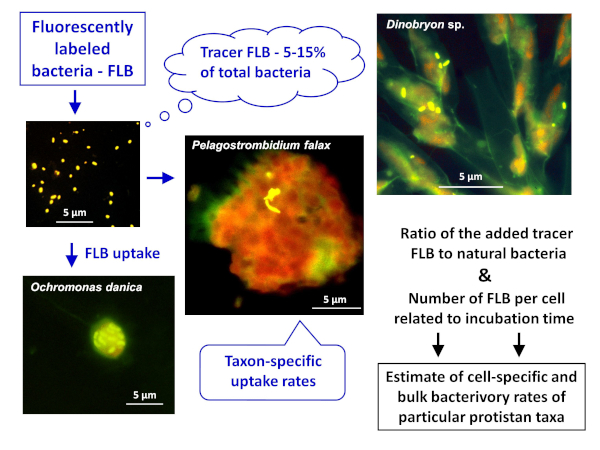
Figure 1: Workflow of using fluorescently labeled bacteria (FLB) to estimate cell- and species-specific grazing rates from the ratio of ingested tracer FLB to total number of natural bacteria in the sample. For more details, see section 7 of the protocol. Please click here to view a larger version of this figure.

Figure 2: Examples of ciliate cells from plankton of a eutrophic fishpond. Examples are shown from the pond with countable FLB in ciliate cells (generally 1-10 tracer FLB per cell, the left two microphotograps) compared to a peritrichous ciliate Pelagovorticella natans (the right side microphotograph). Even during a short, 5 min incubation period, it became "over-labeled" by the tracer FLB, making quantitation of the ingested FLB inaccurate or almost impossible. In this case, it is suggested to decrease the tracer amount to 1.5%-3% of total bacteria. However, generally both the tracer amounts and incubation times can be manipulated to optimize the number of FLB ingested per cell. For more details, see section 7 of the protocol. Please click here to view a larger version of this figure.

Figure 3: Box-and-whisker plots of numbers of FLB per cell of Halteria sp. from the Římov reservoir (Exp I) (A), recalculated to rates of bacterial uptake per hour (B). The data were detected under different seasonal settings, represented by four examples from April to September. The top of panel A shows information on water temperature, different FLB tracers added (%), and different times of sample incubation. It should be noted that the latter two parameters can be modified to optimize number of FLB per cells, with average (full line) and median (dashed line) values approximately between 5-10 FLB per ciliate cell (A). The bars show the 25th and 75th percentiles of all data (50-180 cells inspected) and whiskers stand for the 1th and 99th percentiles. (B) Different small letters indicate significant differences in cell-specific bacterivory rates of Halteria sp. during the studied period. Please click here to view a larger version of this figure.

Figure 4: Box-and-whisker plots of numbers of FLB per cell. Plots are shown of chloroplast-bearing Tetrahymena utriculariae from triplicate treatments of young, mature, and old traps of Utricularia reflexa (Exp II) (A), recalculated to rates of bacterial uptake per hour (C). The data were compared to bacterial uptake rates of the duplicate apochloric populations of T. utriculariae (B,D) isolated from traps but maintained on mixed bacterial suspension growing on wheat grains in dark. On the top of panels A and B, different FLB tracers added (%) and different times of sample incubation are shown. It should be noted that the latter two parameters were modified to optimize number of FLB per cells, with average (full line) and median (dashed line) values approximately between 5-10 FLB per ciliate cell (A,B). The bars show the 5th and 95th percentiles of all data (50-100 cells inspected), and whiskers stand for the 1th and 99th percentiles. Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
Deciphering trophic interaction in aquatic systems is always challenging28, especially at the nano-plankton scales involving protists and their prey, bacteria. When it comes to nutrient uptake pathways and quantification, the application of methods successfully used at higher trophic levels is less possible, due to the high complexity of biotic interactions. These include, for example, stable isotope labeling approaches. This protocol shows the advantages of using epifluorescence microscopy and fluorescently labeled bacteria as a tracer to track and semi-quantify/estimate the carbon flow (bacterial prey: various protistan grazers including mixotrophic grazers29) pathways through the base of microbial food webs. One such advantage is the high accuracy of the single-cell approach, and the other is the unprecedented resolution regarding grazer community structure and distinguishing between different functional guilds, species (Exp I), and even subpopulations of the same species (Exp II).
Critical steps in the protocol
There are several critical steps in the protocol, which can ensure that advantages of the methodology are utilized to their full potential. First, a basic understanding of the studied environment prior to commencement of the experiment is always beneficial. This includes microscopic screening of the diversity and abundance of potential grazers present, bacterial prey sizes, and prey distribution both 1) in the water column (e.g., a vertical profile from the epilimnion to the hypolimnion) and 2) in the case of canyon-shaped reservoirs, on the dam-inflow transect. Second, careful manipulation with collected samples will ensure representative results. Temperature is an extremely important factor affecting most microbial processes12, including protist grazing rates (Figure 3).
Third, manipulating the amount of tracer added based on the quantification of bacterial cells or type of grazer in the sample will ensure that problems with over-labeling (Figure 2) are eliminated. It should be noted that there is a very broad spectrum in ciliate species-specific uptake rates (for details, see step 7.2); thus, to apply the protocol appropriately, prior knowledge of major ciliate species with their time-course uptake rates is essential. It is strongly advised to run preliminary experiments with different tracer amounts to avoid possible ciliate under-labeling (none or too few FLB are taken up per ciliate cell, yielding statistically unsound data) or over-labeling (appears as large numbers of FLB forming "condense FLB clouds" or flocks in ciliate food vacuoles packed by the tracers, thus severely limiting their precise quantification; see upper right example in Figure 2). It should also be noted that incubation times with FLB are generally shorter than 30 min, since the average digestion time of picoplankton by ciliates is around 1.5 h, and digestion starts (ingested picoplankton cells loses its typical shape and color) approximately after 45-60 min30. Similarly, optimal dilution and distribution of samples on the filter prior to microscopic viewing needs to be achieved for accurate results.
Modifications and troubleshooting
The major steps, possible modifications, and troubleshooting modifications of the technique are illustrated in Figure 1 and Figure 2. Additionally, it should be noted that in cases of high concentrations of detrital particles, phytoplankton cells, or their colonies in plankton, such samples 1) should be correspondingly diluted to achieve a stage at which individual grazer cells can be distinguished on the filter surface and 2) be subjected to quantification of food vacuole contents.
Limitations
The main limitation for successful application of this method lies in the presence of various organic detritus or abundant inorganic/organic particles with attached bacteria or aggregates in amounts that prevent clear sample viewing under the epifluorescence microscope and precise estimation of a tracer amount added. It should be noted that the presented tracer technique works primarily with free (i.e., suspended) bacteria that are not attached to particles. However, based on our own experience and literature references (see previous publications2,4,8,10,16,18,21,26), the presented methodology is suitable for most aquatic environments. Examples of two natural, contrasting systems differing in trophic status, detritus content, and grazer diversity and numbers are provided (Figure 3 and Figure 4).
Significance of the approach with respect to existing methods
Importantly, from knowledge of the abundance of a taxon/taxa of bacterivores and their species-specific bacterivory rates, the bulk bacterivory rate of the protistan taxon (or total ciliate assemblage) can be calculated. If this approach is applied to natural plankton environments concomitantly for both heterotrophic flagellates and ciliates (representing the major grazers of bacterioplankton2,6,7), the protistan grazing-induced turnover time of bacterial populations in a given environment can be estimated16,17,18,22. Such data hold fundamental importance for the estimation of carbon flow dynamics in microbial food webs.
Future applications
There are other specific environments in which this method, with some modifications, may be used successfully. These include activated sludge systems, rumen ecosystems, aquatic sediments, and hypertrophic fishponds17. However, application in these nutrient- and microbes-rich environments requires preliminary tests to optimize the protocol regarding the proper size, morphology, and numbers of tracer FLB that can mimic the typical size distribution and other characteristics of prey bacteria inherent to the environment.
Currently, there is increasing interest in combining this approach with the catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH), in which the identity of the grazer cell (e.g., heterotrophic flagellate) is detected with a specific FISH-probe and the uptake rate is based on FLB content in food vacuoles of the flagellate cell on the same microscopic slide31. A sophisticated, new approach called double hybridization32 is a combination of FISH probes at the levels of the predator cell and prey bacteria (that are also labeled specifically by a phylogenetic strain, a bacterial lineage-specific FISH probe). The approach is elegant but also time-consuming and requires specific skills and experience31,32, while application of various FLB uptake approach modifications can be more easily adopted for routine use in laboratories.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by the Czech Science Foundation under the research grant 13-00243S and 19-16554S awarded to K. Š. and D. S., respectively. This article was also supported by the project "Biomanipulation as a tool for improving water quality of dam reservoirs" (No CZ.02.1.01/0.0/0.0/16_025/0007417), funded by the European Regional Development Fund, in Operational Programme Research, Development and Education.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2-µm pore-size filters | SPI supplies, https://www.2spi.com/ | B0225-MB | Black, polycarbonate track etch membrane filters, diameter approprite for filtering apparatus used |
| 5-(4,6-dichlorotriazin-2-yl) aminofluorescein (DTAF) | Any brand | ||
| Automatic pipettes with adjustable volumes | Any brand, various sizes | ||
| Centrifuge | 22 000 x g | ||
| Cryovials | Any brand, 2 mL size | ||
| DAPI (4´,6-Diamidino-2´-phenylindole dihydrochloride) | Any brand | 1 mg ml-1 | |
| Epiflorescence microscope | Magnification from 400 x up to 1000 x | ||
| Filters appropriate for viewing in the DAPI and DTAF range | |||
| Counting grid in one of the oculars | |||
| Filtering apparatus | Usually with a diameter of 25 mm | ||
| Formaldehyde | A brand for microscopy | ||
| Glutaraldehyde | A brand for microscopy | ||
| Immersion oil for microscopy | Specific oil with low fluorescence | ||
| Lugol´s solution | Any brand or see comment | Make an alkaline Lugol' solution as follows: Solution 1 - dissolve 10 g of potassium iodide in 20 ml in MQ water, then add 5 g of iodine. Solution 2 - add 5 g of sodium acetate to 50 ml of MQ water. Add the solution 2 to the solution 1 and thoroughly mix | |
| Methanol stabilized formalin | Any brand available for microscopy purposes | ||
| Microscope slides and cover slips | Any brand produced for microscopy purposes | ||
| MQ water for diluting samples | Any brand |
||
| Phosphate-buffered saline (PBS; pH = 9) | Any brand | 0.05 M Na2HPO4-NaCl solution, adjusted to pH 9 | |
| PPi-saline buffer | Any brand | 0.02 M Na4P2O7-NaCl solution. Add 0.53 g Na4P2O7 to 100 ml of MQ water plus 0.85 g NaCl | |
| Sampling device | Appropriate for obtaining representative sample | e.g. Friedinger sampler for lake plankton | |
| Sodium thiosulfate solution | Any brand | 3% solution is used in the protocol | |
| Sonicator | Any brand | 30 W | |
| Vortex | Any brand allowing thorough mixing of the solutes and samples | ||
| Water bath | Any brand allowing temperature to be maintained at 60 °C |
References
- Azam, F., et al. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series. 10, 257-263 (1983).
- Šimek, K., et al. A finely tuned symphony of factors modulates the microbial food web of a freshwater reservoir in spring. Limnology & Oceanography. 59, 1477-1492 (2014).
- Šimek, K., et al. Bacterial prey food characteristics modulate community growth response of freshwater bacterivorous flagellates. Limnology & Oceanography. 63, 484-502 (2018).
- Šimek, K., et al. Changes in bacterial community composition, dynamics and viral mortality rates associated with enhanced flagellate grazing in a meso-eutrophic reservoir. Applied & Environmental Microbiology. 67, 2723-2733 (2001).
- Jürgens, K., Matz, C. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek. 81, 413-434 (2002).
- Pernthaler, J. Predation on prokaryotes in the water column and its ecological implications. Nature Reviews Microbiology. 3, 537-546 (2005).
- Berninger, U. B., Finlay, J., Kuuppo-Leinikki, P. Protozoan control of bacterial abundances in freshwaters. Limnology and Oceanography. 36, 139-147 (1991).
- Sherr, E. B., Sherr, B. F. Protistan grazing rates via uptake of fluorescently labeled prey. Handbook of Methods in Aquatic Microbial Ecology. Kemp, P. F., Sherr, B. F., Sherr, E. B., Cole, J. J. , Lewis Publishers. Boca Raton, Florida. 695-701 (1993).
- Vazquez-Dominguez, E., Peters, F., Gasol, J. M., Vaqué, D. Measuring the grazing losses of picoplankton: methodological improvements in the use of fluorescently tracers combined with flow cytometry. Aquatic Microbial Ecology. 20, 119-128 (1999).
- Šimek, K., et al. Ecological role and bacterial grazing of Halteria spp.: Small oligotrichs as dominant pelagic ciliate bacterivores. Aquatic Microbial Ecology. 22, 43-56 (2000).
- Montagnes, D. J. S., et al. Selective feeding behaviour of key free-living protists: avenues for continued study. Aquatic Microbial Ecology. 53, 83-98 (2008).
- Kirchman, D. L. Processes in Microbial Ecology. 2nd Edition. , Oxford University Press. Oxford, UK. (2018).
- Porter, K. G., Feig, Y. S. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography. 25, 943-948 (1980).
- Foissner, W., Berger, H. A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology. Freshwater Biology. 35, 375-482 (1996).
- Foissner, W., Berger, H., Schaumburg, J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer Landesamtes für Wasserwirtschaft Heft. , 3-99 (1999).
- Šimek, K., et al. Ciliate grazing on picoplankton in a eutrophic reservoir during the summer phytoplankton maximum: a study at the species and community level. Limnology & Oceanography. 40, 1077-1090 (1995).
- Skibbe, O. An improved quantitative protargol stain for ciliates and other planktonic protists. Archiv für. Hydrobiolgie. 130, 339-347 (1994).
- Macek, M., et al. Growth rates of dominant planktonic ciliates in two freshwater bodies of different trophic degree. Journal of Plankton Research. 18, 463-481 (1996).
- Šimek, K., et al. Microbial food webs in hypertrophic fishponds: omnivorous ciliate taxa are major protistan bacterivores. Limnology & Oceanography. , in press (2019).
- Jezbera, J., et al. Major freshwater bacterioplankton groups: Contrasting trends in distribution of Limnohabitans and Polynucleobacter lineages along a pH gradient of 72 habitats. FEMS Microbiology Ecology. 81, 467-479 (2012).
- Kasalický, V., et al. The diversity of the Limnohabitans genus, an important group of freshwater bacterioplankton, by characterization of 35 isolated strains. PLoS One. 8, 58209 (2013).
- Stabell, T. Ciliate bacterivory in epilimnetic waters. Aquatic Microbial Ecology. 10, 265-272 (1996).
- Zingel, P., et al. Ciliates are the dominant grazers on pico- and nanoplankton in a shallow, naturally highly eutrophic lake. Microbial Ecology. 53, 134-142 (2007).
- Bickel, S. L., Tang, K. W., Grossart, H. P. Ciliate epibionts associated with crustacean zooplankton in german lakes: distribution, motility, and bacterivory. Frontiers in Microbiology. 3 (243), (2012).
- Sirová, D., et al. Hunters or gardeners? Linking community structure and function of trap-associated microbes to the nutrient acquisition strategy of a carnivorous plant. Microbiome. 6, 225 (2018).
- Šimek, K., et al. Ecological traits of a zoochlorellae-bearing Tetrahymena sp. (Ciliophora) living in traps of the carnivorous aquatic plant Utricularia reflexa. Journal of Eukaryotic Microbiology. 64, 336-348 (2017).
- Pitsch, G., et al. The green Tetrahymena utriculariae n. sp. (Ciliophora, Oligohymenophorea) with its endosymbiotic algae (Micractinium sp.), living in the feeding traps of a carnivorous aquatic plant. Journal of Eukaryotic Microbiology. 64, 322-335 (2017).
- Nielsen, J. M., Clare, E. L., Hayden, B., Brett, M. T., Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods in Ecology and Evaluation. 9, 278-291 (2018).
- Beisner, B. E., Grossart, H. P., Gasol, J. M. A guide to methods for estimating phago-mixotrophy in nanophytoplankton. Journal of Plankton Research. , 1-13 (2019).
- Dolan, J. D., Šimek, K. Processing of ingested matter in Strombidium sulcatum, a marine ciliate (Oligotrichida). Limnology and Oceanography. 42, 393-397 (1997).
- Massana, R., et al. Grazing rates and functional diversity of uncultured heterotrophic flagellates. The ISME Journal. 3, 588-596 (2009).
- Grujčić, V., et al. Cryptophyta as major freshwater bacterivores in experiments with manipulated bacterial prey. The ISME Journal. 12, 1668-1681 (2018).



