16.10: Titration of a Polyprotic Acid
A polyprotic acid contains more than one ionizable hydrogen and undergoes a stepwise ionization process. If the acid dissociation constants of the ionizable protons differ sufficiently from each other, then the titration curve for such polyprotic acid generates a distinct equivalence point for each of its ionizable hydrogens. Therefore, titration of a diprotic acid results in the formation of two equivalence points, whereas the titration of a triprotic acid results in the formation of three equivalence points on the titration curve.
Carbonic acid, H2CO3, is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts.
First ionization:
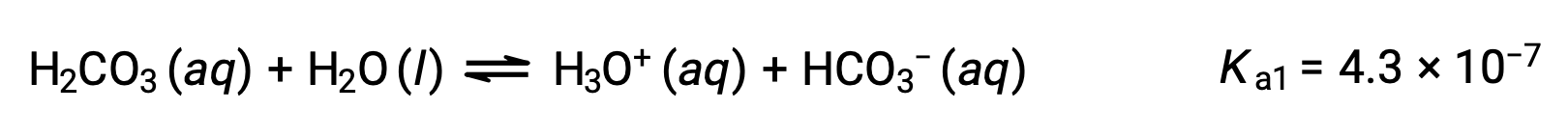
The bicarbonate ion can also act as an acid. It ionizes and forms hydronium ions and carbonate ions in even smaller quantities.
Second ionization:
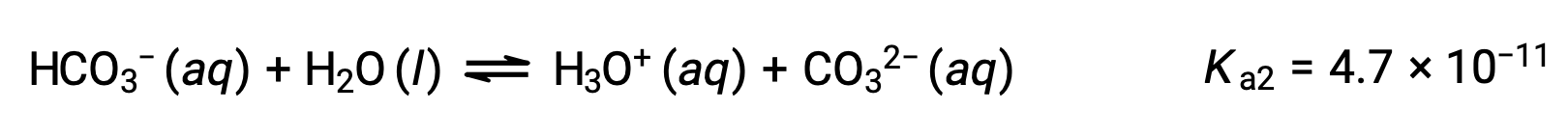
The Ka1 is larger than the Ka2 by a factor of 104. Therefore, when H2CO3 is titrated with a strong base like NaOH, it produces two distinct equivalence points for each ionizable hydrogen.
Phosphoric acid, a triprotic acid, ionizes in three steps:
First ionization:
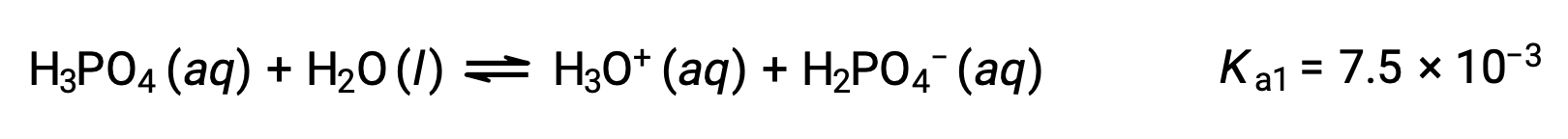
Second ionization:
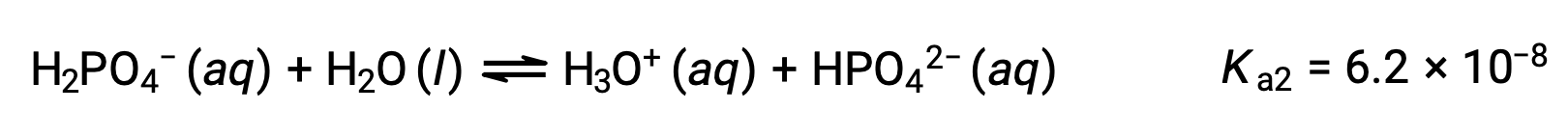
Third ionization:
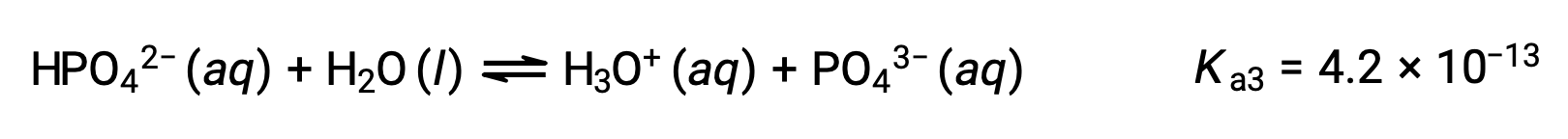
When H3PO4 is titrated with a strong base like KOH, it produces three equivalence points for each ionizable hydrogen. However, as HPO42− is a very weak acid, the third equivalence point is not easily discernible on the titration curve.
This text is adapted from Openstax, Chemistry 2e, Section 14.5: Polyprotic Acids.


