Summary
Acidic postconditioning protects against cerebral ischemia. Here we present two models to execute APC. They are achieved respectively by transferring corticostriatal slices to acidic buffer after oxygen-glucose deprivation in vitro and by inhaling 20% CO2 after middle cerebral artery occlusion in vivo.
Abstract
Stroke is one of the leading causes of mortality and disability worldwide, with limited therapeutic approaches. As an endogenous strategy for neuroprotection, postconditioning treatments have proven to be promising therapies against cerebral ischemia. However, complicated procedures and potential safety issues limit their clinical application. To overcome these disadvantages, we have developed acidic postconditioning (APC) as a therapy for experimental focal cerebral ischemia. APC refers to the mild acidosis treatment by inhaling CO2 during reperfusion following ischemia. Here we present two models to execute APC in vitro and in vivo, respectively. The oxygen-glucose deprivation (OGD) treatment of mice and the corticostriatal occlusion and middle cerebral artery occlusion (MCAO) of mice were employed to mimic cerebral ischemia. APC can be simply achieved by transferring brain slices to acidic buffer bubbled with 20% CO2, or by mice inhaling 20% CO2. APC showed significant protective effects against cerebral ischemia, as reflected by tissue viability and brain infarct volume.
Introduction
Stroke is one of the leading causes of mortality and disability worldwide. Great efforts have been made to find effective treatments for stroke in the last decades, however, the achievement is quite unsatisfactory. Postconditioning is a process manipulated by subtoxic stresses following an ischemic episode. Postconditioning, including ischemic, hypoxic, low-glucose and remote ischemic postconditioning, trigger endogenous adaptive mechanisms, and have been proven to be promising therapies against cerebral ischemia1,2,3,4. However, ischemic postconditioning may introduce additional injury. Limb remote ischemic postconditioning usually needs several cycles of 5 - 20 min occlusion and reperfusion on the ipsilateral or bilateral hind limbs5,6,7. Therefore, these postconditioning manipulations are dangerous or impractical in clinical practice. To overcome these disadvantages, we have developed APC as a therapy for focal cerebral ischemia in mice8. Induced simply by inhaling 20% CO2, APC significantly reduces ischemic brain injury in a more feasible and safer way. Recently we have proved that APC extends the reperfusion window, highlighting the significance of APC for stroke therapy9.
Here we present two experimental models to study the neuroprotection of APC against cerebral ischemia. The first one is the oxygen-glucose deprivation (OGD) model in mice corticostriatal slices. Rapid preparation and transfer of the brain slices into an artificial environment, usually artificial cerebrospinal fluid (ASCF), can maintain cell viability and neuronal circuitry, which makes it possible to study brain function in vitro10,11. OGD in ASCF mimics cerebral ischemia and induces ischemic injury12,13,14. After OGD, the brain slices are refreshed in regular ASCF (r-ASCF) to provide reperfusion and then treated with APC using acidic ASCF bubbled with 20% CO2. The corticostriatal slice maintains the intact histological characterization compared with primary cultured cells.
To study brain function in vivo, the mouse middle cerebral artery occlusion (MCAO) model is employed. The middle cerebral artery is blocked by inserting a flame-blunted monofilament via the common carotid artery. As one of the most widely used stroke models, the MCAO model shows clinical relevance and the application of a monofilament makes it easier to achieve reperfusion. Simply by inhaling normoxic mixed gas containing 20% CO2 after the onset of reperfusion, APC showed significant protective effects against cerebral ischemia indicated by reduced brain infarct volumes.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
All experiments were approved by and conducted in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize any pain or discomfort, and the minimum number of animals was used.
1. OGD of Corticostriatal Slices
- Solution preparation:
- Prepare 1,000 mL r-ACSF (124 mmol/L, NaCl, 5 mmol/L KCl, 1.25 mmol/L KH2PO4, 2 mmol/L MgSO4, 26 mmol/L NaHCO3, 2 mmol/L CaCl2, 10 mmol/L glucose, final pH 7.4). Bubble with 5% CO2 and 95% O2 throughout the experiment.
- Prepare 200 mL glucose-free ACSF (gf-ACSF) as above but excluding glucose, bubbled with 5% CO2 and 95% N2. Bubble the gases for 30 min before applying to brain slices to reduce the oxygen content in the solution. Continue bubbling until the OGD procedure is finished.
- Prepare acidic ACSF (a-ACSF) by equilibrating 200 mL r-ACSF with 20% CO2 and 80% O2. Bubble the gases for 30 min before applying to brain slices to reduce the pH of the solution to pH 6.8, and continue bubbling until APC procedure is finished.
- Place three tissue holders into r-ACSF, gf-ACSF, a-ACSF, respectively.
- Brain slice preparation:
Note: 8-week-old, male C57Bl/6J mice were used in this study. The animals were housed under controlled temperature (22 ± 2 °C), with a 12 h light-dark cycle period and access to pelleted food and water.- Sacrifice a mouse with an isoflurane overdose in an induction chamber. Decapitate the mouse. Dissect out the brain using small scissors and forceps. Remove the brain with a thin spatula and drop it carefully into a glass beaker containing ice-cold r-ACSF equilibrated with 5% CO2 and 95% O2.
NOTE: These steps should be done as quickly but meticulously as possible. - Keep the brain in the ice-cold r-ACSF for 5 min. Adjust the gas pressure to avoid movements of the brain.
- Place cryoprecipitate glue on the vibratome plate in two strips. Put a piece of 3% agarose upon the glue strip away from the blade to support the brain.
- Invert a 10-cm Petri dish on ice and then put a filter paper on the dish. Moisten the filter paper with drops of ice-cold r-ACSF.
- Transfer the brain to the filter paper with forceps. Cut off the frontal pole and the cerebellum with blade and forceps.
NOTE: The transverse sections should be as smooth as possible and vertical to sagittal plane. - Place the remaining brain tissue vertically upon the other strip of glue and keep the brain leaning against the agarose. Add ice-cold r-ACSF to the cutting reservoir to submerge the brain and add ice to the ice holder area. Keep the r-ACSF in the reservoir bubbled with 5% CO2 and 95% O2.
- Raise the reservoir and adjust the position of the razor to place the razor as close to the brain as possible and just above the brain.
- Choose "CONT" mode to slice the brain sections continuously. Set the cutting thickness to 400 µm, vibrating frequency to 6 - 8, and speed to 3 - 4. Press the "start/stop" button to start automatic cutting.
- Cut off the tip of a 3 mL Pasteur pipette to make an opening matching the size of the brain slices.
NOTE: The opening should be large enough to avoid additional damage to the brain slices. - Draw out five brain slices one by one with the tip-cutPasteur pipette and place them into the tissue holder in ice-cold r-ACSF bubbled with 5% CO2 and 95% O2. Do not collect the first slice produced. Adjust the gas pressure to avoid movements of the brain slices.
NOTE: The brain slices should not cover each other. - Place brain slices with r-ACSF at room temperature for 30 min and then at 37 °C in a water bath for another 10 min to recover synaptic functions.
- Sacrifice a mouse with an isoflurane overdose in an induction chamber. Decapitate the mouse. Dissect out the brain using small scissors and forceps. Remove the brain with a thin spatula and drop it carefully into a glass beaker containing ice-cold r-ACSF equilibrated with 5% CO2 and 95% O2.
- OGD and APC:
- Place gf-ACSF and a-ACSF into a 37 °C water bath and bubble the buffers with the corresponding gases as mentioned above in 1.1.2 and 1.1.3 for 30 min.
- Leave one slice in r-ACSF as a control. Transfer the other four brain slices into the tissue holder with preheated gf-ACSF carefully and incubate for 15 min. Transfer the slices back into the tissue holder in r-ACSF to achieve reperfusion.
- To study the time window of APC, transfer one slice directly from gf-ACSF to a-ACSF. Transfer the other three slices to r-ACSF at first, and then transfer two of them to the tissue holder in a-ACSF 5 and 15 min after reperfusion respectively.
- Incubate the two slices in a-ACSF for 3 min and then transfer them back to r-ACSF. Incubate the three slices in r-ACSF for another 1 h. Set the remaining slices in r-ACSF as the OGD group.
- For a dose response study, transfer all four slices to the tissue holder in r-ACSF at first after OGD and incubate for 5 min. Then leave one slice in r-ACSF as the OGD group and transfer the other three slices to a-ACSF. Incubate the slices in a-ACSF for 1, 3 and 5 min respectively and then transfer them back to r-ACSF. Incubate the three slices in r-ACSF for another 1 h.
- Slice viability determination:
- Prepare 0.25% 2,3,5-triphenyltetrazolium hydrochloride (TTC) normal saline solution. Add 1.25 g TTC powder to 500 mL normal saline solution.
NOTE: After the powder completely dissolves, transfer the solution into a 24-well plate (500 µL per well) covered in foil and store it at 4 °C. TTC and tissue stained with TTC are light sensitive. - Transfer the slices into a 24-well plate (one slice per well) containing TTC solution and stretch out the slice in the solution. Incubate the slices in TTC solution at 37 °C in a shallow water bath for 30 min.
- Transfer the slices into clean 1.5 mL centrifuge tubes covered in foil and measure the dry weight of the slices.
- Add ethanol/dimethyl sulfoxide (1:1) into the centrifuge tubes (v:w = 10:1) to extract formazan. Incubate the slices in ethanol/dimethyl sulfoxide at room temperature away from light for 24 h.
- Add all ethanol/dimethyl sulfoxide in the tubes into a 96-well plate and measure the absorbance at 490 nm by a plate reader.
- Normalize the absorbance to the dry weight of the slice and express viability as the percentage of control slice.
- Prepare 0.25% 2,3,5-triphenyltetrazolium hydrochloride (TTC) normal saline solution. Add 1.25 g TTC powder to 500 mL normal saline solution.
2. MCAO
- Preparation:
- Disinfect the workbench, the surface of the Laser Doppler Flowmetry (LDF) instrument and its accessories with 70% ethyl alcohol.
- Prepare autoclaved instruments: two scissors, 10 cm; two forceps, 10 cm; one ophthalmic forceps, 11 cm; one micro ophthalmic scissors, 9 cm; one microvessel clamp, 1.8 cm; oneneedle drive,r 12.5 cm; several surgical sutures (△ 1/2 4 × 10); cotton swabs.
- Prepare a syringe (without needle) with saline solution to maintain a hydrated operation area.
- Prepare the anesthesia gas (100% O2 + isoflurane).
- Detection of brain blood flow:
- Set up the LDF instrument.
- Inject analgesics intraperitoneally into the mouse: metamizol 200 mg/kg, carprofen 4 mg/kg and buprenorphin 0.1 mg/kg.
- Place the mouse into an induction chamber with 4% isoflurane in oxygen to anesthetize it until spontaneous movement of body and vibrissae stops.
- Place the mouse in a prone position with its nose fitted into the nose cone and maintain isoflurane at 1.5% for the subsequent procedure.
- Apply dexpanthenol eye ointment on both eyes.
- Disinfect the head with 70% ethyl alcohol.
- Make a right paramedian skin incision between eyes and ears using a scalpel to expose the bregma.
- Rub the skull with sterile cotton.
- Apply one to two drops of surgical glue to the surface of the skull.
- Place the tip of fiber optic probe 5 mm caudal to the bregma and 6 mm lateral to the midline.
- Start recording the blood flow on the computer software. Wait until a stable blood flow baseline occurs and then apply a 20 µL catalyst upon the glue to fix the probe.
NOTE: An ideal baseline should be more than 500 flux. - If the baseline is less than 200 flux, adjust the position finely to get a suitable baseline. Keep the probe attached to the skull for the duration of the experiment.
- Disinfect the head with iodophor after the fiber optic probe attached.
- MCAO:
- Place and fix the anesthetized mouse (with LDF probe attached to its skull) in a supine position and maintain its body temperature using a heat lamp throughout the surgery. Disinfect the neck with 70% ethyl alcohol.
- Make a paramedian skin incision in the neck using a scalpel; blunt dissect the soft tissues with forceps to expose vessels. Add one drop of saline to the exposed tissues to keep themhydrated.
- Dissect the common carotid artery from surrounding tissue and the vagus nerve using ophthalmic forceps. Be careful not to damage the vagus nerve. Disinfect skin of neck again with iodophor after the common carotid artery exposed.
- Place a microvessel clamp at distal end of the common carotid artery and then tie a dead knot with 6-0 silk sutures at the proximal end. Place the clamp as proximal as possible for subsequent operations. Ensure that the length of the vessel between the clamp and dead knot is as long as possible for subsequent operations.
- Make a temporary suture proximal to the clamp by tying a loose knot. Ensure that there is enough space between two knots for monofilament insertion.
- Make a smalllongitudinal incision between the two knots with micro ophthalmic scissors. Ensure the incision is as close to the dead knot as possible for subsequent operations. Be careful not to cut off the vessel.
- Insert a 12-mm tip-blunted monofilamentthrough the incision to enter the artery lumen and advance it a few mm. Tighten the loose knot around the tip of the monofilament and then remove the clamp.
- Advance the filament into the internal carotid artery (10 mm to occlude the middle cerebral artery) with ophthalmic forceps until the LDF software shows a sharp decline (>80% reduction from baseline blood flow) in blood flow. Record the start time of occlusion.
NOTE: LDF instrument setup is described in section 2.2. - Occlude the middle cerebral artery for 1 h. Cut off the LDF probe and put the mice in a 30 °C incubator for the duration of occlusion.
- Reperfusion and APC:
- Re-anesthetize the animals with isoflurane as described above 55 min after the beginning time of occlusion. Place and fix mice in a supine position. Open the neck incision and re-expose the common carotid artery.
- Gently pull out the filament with ophthalmic forceps after occlusion period to achieve reperfusion and turn the temporary suture into a permanent one by tightening the knot.
- For acidosis treatment, change the gas inhaled by the nose cone to the mix gases containing 20% CO2, 20% O2 and 60% N2 for 5 min at 5, 50, or 100 min after reperfusion. Close the incision with an interrupted surgical suture.
- Place mice in a 30 °C heat cage until mice recover consciousness and then return the mice to clean, individual cages. Provide mice with a Petri dish of water and moistened food. Monitor the mice closely after the surgery for excessive pain and death.
- Brain Infarct volume measurement:
- Measure brain infarct volume by usingTTC staining 24 h after reperfusion.
- Prepare 0.25% TTC solution as mentioned in step 1.4.1 prior to the designated time of sacrifice. Transfer the solution into a 24-well plate (1 mL per well) covered in foil and store it at 4 °C.
NOTE: TTC and tissue stained with TTC are light sensitive. - At 24 h after reperfusion, sacrifice the animal with an isoflurane overdose in an induction chamber. Decapitate the mice. Dissect out the brains using small scissors and forceps. Examine blooding points on the brains to exclude the mice that underwent subarachnoid hemorrhage at the Circle of Willis.
- Place the brain on a clean glass slide on a -20 °C ice pack. Place the brain and the glass slide in a -20 °C fridge for 5 min to make the brain easier to slice.
- Take out the brain and the glass slide from -20 °C and put them back on the -20 °C ice pack. Dissect the frontal pole and the cerebellum with blade and forceps.
- Slice the brain sections horizontally to a 1-mm thickness with a blade to produce 5 slices. Transfer the slices into the 24-well plate containing TTC solution (1 slice per well) with forceps and stretch out the slices in the solution. Incubate the slices in TTC solution at 37 °C in a shallow water bath for 30 min.
- Aspirate the TTC solution. Add 10% formaldehyde (1 mL per well) and incubate at room temperature for 30 min. Place the slices in the order in which they were cut upon a cellophane sheet and scan the segments into the photographic plate imager.
- Analyze the infarct size as a percentage of the whole brain slice using ImageJ analysis software8based on visual identification; see Figure 2 (left).
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
In the corticostriatal slice model described above, corticostriatal slice viability was quantified by TTC assay at 1 h after reperfusion. TTC conversion was calculated by normalizing the absorption at 490 nm to the control slice. According to TTC conversion, APC protected against OGD-induced reperfusion injury in an onset time and duration-dependent manner. In detail, both 1 and 3 min of acidosis treatment significantly improved viability at 5 min after 15 min OGD, whereas 5 min did not (OGD: 0.609 ± 0.029, 5/1: 0.758 ± 0.034, 5/3: 0.821 ± 0.041, 5/5: 0.672 ± 0.053, data reported as mean ± SEM) (Figure 1A). The neuroprotection by acidosis treatment remained protective within 5 min after reperfusion, whereas 15 min did not (OGD: 0.584 ± 0.044, 0/3: 0.762 ± 0.036, 5/3: 0.833 ± 0.062, 15/3: 0.627 ± 0.038) (Figure 1B).
In the MCAO model, 5 min of acidosis treatment initiated at 5 min after the onset of reperfusion rescued cell death caused by ischemic insult, reflected by smaller infarct volumes (MCAO: 33.4 ± 4.4%, 5/5: 16.6 ± 2.7%, 50/5: 19.5 ± 2.1%, 100/5: 37 ± 2.1%). The neuroprotection was still robust, even when the onset time was delayed to 50 min after reperfusion. However, acidosis treatment initiated at 100 min did not block the ischemic injuries (Figure 2).
All data were collected and analyzed in a blinded fashion. Data are presented as mean ± SEM. In the corticostriatal slice model, each group has 6-8 samples. In the MCAO model, each group has 9 - 10 samples. One-way analysis of variance with least significant difference was applied for multiple comparisons.
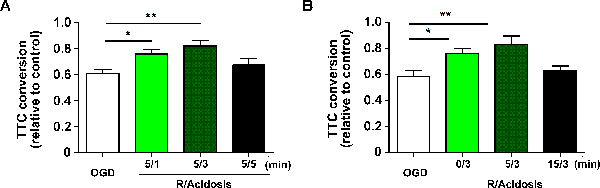
Figure 1. APC Protects Against OGD-induced Reperfusion Injury in Corticostriatal Slices. Corticostriatal slices were treated with acidosis (pH 6.8) for indicated durations (A) and indicated recovery periods (B) after OGD. The cell viability was assessed by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay at 24 h after reperfusion, and corticostriatal slice viability was quantified by TTC assay at 1 h after reperfusion. Values show mean ± SEM. n= 6 - 8 for each group; *p< 0.05 and **p< 0.01 by one-way analysis of variance; R, reperfusion. Please click here to view a larger version of this figure.
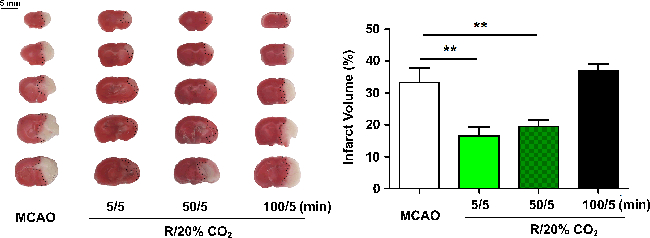
Figure 2. APC Protects Against MCAO-induced Injury in Mice. Animals were subjected to 60 min MCAO and treated by inhaling 20% CO2 for 5 min at 5, 50, or 100 min after reperfusion. Infarct volume was quantified by 2,3,5-triphenyltetrazolium hydrochloride staining at 24 h after reperfusion (indicated by black dotted line on the left panel). Values show mean ± SEM. n = 8 - 10 for each group; *p< 0.05 and **p< 0.01 by one-way analysis of variance; R, reperfusion. Please click here to view a larger version of this figure.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
Here we present two experimental models to study the neuroprotection of APC against cerebral ischemia. In brain slices, APC is achieved by incubating mice corticostriatal slices in acidic buffer bubbled with 20% CO2 after reperfusion onset, while in the MCAO model, APC is achieved by inhaling 20% CO2 to mice after reperfusion. Both models reflect the neuroprotection of APC against cerebral ischemia. The protection was comparable with that achieved by ischemic postconditioning but with a wider time window. In the MCAO model, the time window could be as long as 50 min after reperfusion compared to 10 min for ischemic postconditioning15. In addition, the procedure of APC is easier and safer.
In the corticostriatal slices model, gentle and rapid operations are crucial to warrant the viability of brain slices to the maximum extent. For the MCAO performance, the cerebral blood flow monitoring is required, and the sharp decline of blood flow must be observed to ensure the occlusion of middle cerebral artery. After the brains were dissected out for TTC staining, careful examination of the brains is necessary for exclusion of subarachnoid hemorrhage.
The extension and the duration of acidosis are critical to achieve the neuroprotection of APC. Prolonged duration of acidosis treatment is not beneficial in corticostriatal slices model (Figure 1A). In addition, our previous study showed that both 10% and 20% CO2 inhalation, rather than 30% CO2 inhalation confer neuroprotection on mice8. These suggest mild acidosis is crucial for the neuroprotection of APC, and thus the concentration of CO2 and the duration of APC are indispensable for acidosis neuroprotection.
In addition to TTC staining, many techniques can be combined to satisfy diverse research needs. For instance, extra-cellular electric recording can be added to the final step to observe how ischemia and acidosis affect neural potential16. The perfusion solution of brain slice can also be collected to measure the concentration change of amino acid neurotransmitters after APC by HPLC17. Slices of a certain brain regions can be prepared to study the responses of a particular area to ischemia and APC. Overall, many alternatives can be introduced to illuminate the impacts of ischemia and acidosis.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81573406, 81373393, 81273506, 81221003, 81473186 and 81402907), Zhejiang Provincial Natural Science Foundation (LR15H310001) and the Program for Zhejiang Leading Team of S&T Innovation Team (2011R50014).
Materials
| Name | Company | Catalog Number | Comments |
| Sodium chloride | Sigma | S5886 | |
| Potassium chloride | Sigma | P5405 | |
| Potassium phosphate monobasic | Sigma | P9791 | |
| Magnesium sulfate | Sigma | M2643 | |
| Sodium bicarbonate | Sigma | S5761 | |
| Calcium chloride dihydrate | Sigma | C5080 | |
| D-(+)-Glucose | Sigma | G7021 | |
| Vibratome | Leica | VT1000 S | |
| 2,3,5-triphenyltetrazolium hydrochloride | Sigma | T8877 | |
| Absolute Ethanol | Aladdin Industrial Corporation | E111993 | |
| Dimethyl sulfoxide | Sigma | D8418 | |
| Laser Doppler Flowmetry | Moor Instruments Ltd | Model Moor VMS-LDF2 | |
| Diethyl ether anhydrous | Sinopharm Chemical Reagent Corporation | 80059618 | |
| Trichloroacetaldehycle hydrate | Sinopharm Chemical Reagent Corporation | 30037517 | |
| 10% Formalin | Aladdin Industrial Corporation | F111936 | |
| 24-well plates | Jet Biofil | TCP-010-024 |
References
- Zhao, H., Sapolsky, R. M., Steinberg, G. K. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 26 (9), 1114-1121 (2006).
- Leconte, C., et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 40 (10), 3349-3355 (2009).
- Fan, Y. Y., et al. Transient lack of glucose but not O2 is involved in ischemic postconditioning-induced neuroprotection. CNS Neurosci Ther. 19 (1), 30-37 (2013).
- Hess, D. C., Hoda, M. N., Bhatia, K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke. Stroke. 44 (4), 1191-1197 (2013).
- Ren, C., Yan, Z., Wei, D., Gao, X., Chen, X., Zhao, H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 1288, 88-94 (2009).
- Sun, J., et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab. 32 (5), 851-859 (2012).
- Li, P., et al. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int J Neurosci. , 1-8 (2015).
- Fan, Y. Y., et al. A novel neuroprotective strategy for ischemic stroke: transient mild acidosis treatment by CO2 inhalation at reperfusion. J Cereb Blood Flow Metab. 34 (2), 275-283 (2014).
- Shen, Z., et al. PARK2-dependent mitophagy induced by acidic postconditioning protects against focal cerebral ischemia and extends the reperfusion window. Autophagy. 0, (2017).
- Skolnik, J., Takacs, L., Szende, E. In vitro oxygen consumption of slices from kidney, brain, cortex and liver in hypoxia. Nature. 209 (5020), 305 (1966).
- Lynch, G., Schubert, P. The use of in vitro. brain slices for multidisciplinary studies of synaptic function. Annu Rev Neurosci. 3, 1-22 (1980).
- Zheng, S., Zuo, Z. Isoflurane preconditioning reduces purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 118 (1), 99-106 (2003).
- Yin, B., Barrionuevo, G., Weber, S. G. Optimized real-time monitoring of glutathione redox status in single pyramidal neurons in organotypic hippocampal slices during oxygen-glucose deprivation and reperfusion. ACS Chem Neurosci. 6 (11), 1838-1848 (2015).
- Medvedeva, Y. V., Ji, S., Yin, H. Z., Weiss, J. H. Differential vulnerability of CA1 vs CA3 pyramidal neurons after ischemia: possible relationship to sources of Zn2+ accumulation and its entry into and prolonged effects on mitochondria. J Neurosci. , (2016).
- Pignataro, G., et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 28 (2), 232-241 (2008).
- Zhang, X., Ding, H. Z., Jiang, S., Zeng, Y. M., Tang, Q. F. An in vitro study of the neuroprotective effect of propofol on hypoxic hippocampal slice. Brain Inj. 28 (13-14), 1758-1765 (2014).
- Niu, Y., et al. Chemical profiling with HPLC-FTMS of exogenous and endogenous chemicals susceptible to the administration of chotosan in an animal model of type 2 diabetes-induced dementia. J Pharm Biomed Anal. 104, 21-30 (2015).



