Summary
To improve our knowledge of cellular and molecular neotissue formation, a murine model of the TEVG was recently developed. The grafts were implanted as infrarenal vena cava interposition grafts in C57BL/6 mice. This model achieves similar results to those achieved in our clinical investigation, but over a far shortened time-course.
Abstract
Biodegradable scaffolds seeded with bone marrow mononuclear cells (BMCs) are often used for reconstructive surgery to treat congenital cardiac anomalies. The long-term clinical results showed excellent patency rates, however, with significant incidence of stenosis. To investigate the cellular and molecular mechanisms of vascular neotissue formation and prevent stenosis development in tissue engineered vascular grafts (TEVGs), we developed a mouse model of the graft with approximately 1 mm internal diameter. First, the TEVGs were assembled from biodegradable tubular scaffolds fabricated from a polyglycolic acid nonwoven felt mesh coated with ε-caprolactone and L-lactide copolymer. The scaffolds were then placed in a lyophilizer, vacuumed for 24 hr, and stored in a desiccator until cell seeding. Second, bone marrow was collected from donor mice and mononuclear cells were isolated by density gradient centrifugation. Third, approximately one million cells were seeded on a scaffold and incubated O/N. Finally, the seeded scaffolds were then implanted as infrarenal vena cava interposition grafts in C57BL/6 mice. The implanted grafts demonstrated excellent patency (>90%) without evidence of thromboembolic complications or aneurysmal formation. This murine model will aid us in understanding and quantifying the cellular and molecular mechanisms of neotissue formation in the TEVG.
Introduction
Congenital heart defects are serious conditions that affect nearly 8% of live births in the United States. Approximately 25% of those infants with congenital heart defects or 2.4 per 1,000 live birth, require invasive treatment in the first year of their life1. The most effective treatment for congenital heart disease is reconstructive surgery. Unfortunately, complications arising from the use of currently available vascular conduits are the most significant cause of postoperative morbidity and mortality.
To address this problem, we developed the first tissue engineered vascular grafts (TEVGs) for clinical use2. TEVGs were constructed from biodegradable polyester tubes seeded with autologous bone marrow derived-mononuclear cells (BM-MNCs) and implanted as venous conduits for congenital heart surgery. The results showed excellent patency rates at 1-3 years of follow-up, but with significant incidence of stenosis3,4. It was clear that a better understanding of vascular neotissue formation and the mechanism underlying the development of TEVG stenosis was needed. In order to better understand the development of TEVGs and the mechanism of stenosis development, an ovine model was created5,6. In this model, the TEVGs successfully transformed into living vessels and were similar in both morphology and function of native veins. This use of a large animal model was a good first step in providing important pre-clinical information that aided clinical usage of TEVGs. However, full understanding of the cellular and molecular mechanisms of vascular neotissue formation in TEVGs using large animal models is limited due to limitations in molecular characterization of vascular cell phenotypes due to lack of species specific molecular tools. To overcome these shortcomings, a murine model of TEVGs was developed by reason of the rapid advancement in mouse genetics and their extensive molecular characterization with the added advantage of a shortened time scale.
The murine IVC interposition model faithfully recapitulated the process of neovessel formation that occurs in large animals and humans, but over a much shorter time course6-9. Here, a detailed protocol for small-scale graft manufacturing using biodegradable scaffolds, BM-MNC harvesting and isolation, BM-MNC seeding on scaffold, and graft implantation in a murine model were described.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
NOTE: All animal procedures were approved by the Nationwide Children's Hospital Institutional Animal Care and Use Committee.
1. Graft Manufacturing
- Make the ε-caprolactone and L-lactide copolymer P(LA/CL) solution by adding 100mg P(LA/CL) in 2 ml dioxane under a fume hood. Place the solution on a vortex and mix continuously for 1-1.5 hr to dissolve completely.
- In the meantime, remove a sheet of polyglycolic acid (PGA) felt from the freezer and cut out several 5 x 8 mm sections. Also cut off the tip of a 0.1-10 µl pipette just above the filter.
- Insert a 19 G needle (1.5 length) into the distal end of a pipet tip and wrap the PGA felt around the needle using micro forceps.
- Carefully push the felt to the distal end of the pipet tip, where the lumen is straighter than the proximal part, using a blunt 18 G needle, while the 19 G needle is inserted into it.
- Pipette 40 µl P(CL/LA) solution into the pipette tip from the top. Saturate the PGA felt with the solution. Then push air bubbles out using a pipette dispenser. Repeat this process if needed.
- Place grafts in a 50 ml tube and place it in a -80 °C freezer for 20 min. Make sure the head of the needles face downward.
- Transfer the tube into a lyophilizer and vacuum for 24 hr. Make sure to open the lid of the tube to have airflow.
- Take out the grafts and remove them from the needles. Cut both ends of the graft leaving a ~5 mm section and put them back onto the needles to maintain their shape. Keep the grafts in a desiccator.
- Place the scaffold under UV light in a biosafety hood O/N before cell seeding.
2. Bone Marrow Mononuclear Cell Harvesting and Isolation
- Euthanize the mice with ketamine/xylazine overdose (ketamine, 200 mg/kg and xylazine, 20 mg/kg).
- Remove bones (femurs and tibias) from 3 mice for 10 graft implantations and place in a Petri dish with 10 cc RPMI. Cut both ends of the bones and flush bone marrow using a syringe with a 25 G needle into a new Petri dish with 3 cc RPMI. Collect bone marrow and RPMI solution in a 15 cc tube and wash the petri dish with additional 2 cc RPMI to collect the remainder of BM.
- Take sample (5-10 µl) and count cell using an automated cell counter or hemocytometer. Record the result.
- Put 5 cc Ficoll in a 15 cc centrifuge tube and add bone marrow and RPMI solution. Add the solution very gently to prevent it from mixing with Ficoll.
- Centrifuge at 528 x g for 30 min with "NO BRAKE" at 24 °C.
- Remove the upper pink layer. Collect the middle clear layer Figure 1, which is the MNC layer, and dilute it with PBS 1:1.
- Centrifuge the dilute MNC solution at 528 x g for 10 min at 24 °C.
- Remove the supernatant and dilute the pellet with 5 ml PBS.
- Centrifuge the pellet solution at 528 x g for 10 min at 24 °C.
- Remove the supernatant. Dilute the pellet with the appropriate volume of RPMI (~200 µl).
- Take a 5-10 µl sample and count cells using an automated cell counter or hemocytometer. Record the result. Repeat the cell counting one more time and calculate the average cell number.
- Dilute cell concentration to 1,000,000 cells/10 µl using RPMI.
3. Cell Seeding
- Prewet scaffold by adding 5 µl RPMI luminally for 5 min, then remove RPMI.
- Add 10 µl bone marrow derived mono nuclear cells in RPMI from Step 2.12 to scaffold lumen and wait for 10 min to allow cells to attach onto the scaffold.
- Cut a 19 G needle to 1 cm length and put the needle into the lumen of the scaffold to keep the shape of the scaffold. Place the sample in a 24-well plate.
- Add 1,000 µl RPMI to each well and incubate O/N in an incubator.
4. Graft Implantation
- Autoclave all the surgical tools before the surgery: 1x fine scissors, 3x micro forceps, 2x micro vascular clamps, 1x clamp applying forceps, 1x micro needle holder, 1x spring scissors, 1x retractor.
- 6-8 weeks old female C57BL/6 are used as tissue engineered vascular graft recipients. Remove the mouse from its cage and weigh it, then anesthetize through an intraperitoneal injection into the lower right quadrant of the abdomen with a ketamine/xylazine cocktail (ketamine, 100 mg/kg and xylazine, 10 mg/kg). Ketoprofen (5 mg/kg, IP) is used as a pre-anesthesia analgesic.
- Check the level of sedation by tale pinching, then clip the abdominal hair. Lubricate the eyes with sterile ophthalmic ointment, and place the mouse in a dorsal recumbence position on a pad. Disinfect the abdomen with betadine and alcohol pads. Cover the mouse with a sterile drape and expose the incision area only.
- Make a midline laparotomy incision from below the xyphoid to the suprapubic region, and insert a self-retaining retractor. Wrap the intestines in saline moistened gauze. Bluntly define the infrarenal aorta and vena cava.
- Place two micro vascular clamps on both proximal and distal sides of the aorta and vena cava then bluntly separate the aorta from the vena cava Transect the vena cava. If necessary, ligate the abdominal aortic branches with a 10-0 monofilament sutures on tapered needles.
- Implant an inferior vena cava interposition graft with proximal and distal end to end anastomoses using a sterile 10-0 suture. Trim the graft, usually 1-2 mm, dependent on the anatomy of the mouse. Secure the graft with one stitch on both proximal and distal ends and start to suture continuously with 4-5 stiches from the other side of the graft. After finishing the front side, flip the clamps and grafts to the other side and suture the back side of the graft. During implantation, flush the graft with heparin solution frequently to prevent acute thrombosis.
- Remove the proximal clamp and control the hemorrhage by applying a topical absorbable sterile hemostat agent. When the hemorrhage stops completely, remove the distal clamp and control the hemorrhage the same way. Make sure blood flows through the graft.
- Close the abdominal musculature and skin in two layers using a 6-0 black polyamide monofilament suture with included threaded needle.
- Inject 0.5 ml saline subcutaneously and place the mouse in a recovery cage on a warming pad until the mouse is fully mobile. Upon recovery, return the mouse to a new cage with paper bedding. Give pain medication (Ibuprofen, 30 mg/kg, drinking water) for 48 hr.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
A schematic of TEVG implantation is shown in Figure 1. Bone marrow was harvested from a donor mouse and mono nuclear cells were isolated using density centrifugation and then seeded onto a biodegradable scaffold. The seeded scaffolds were incubated O/N and implanted to a recipient mouse as an inferior vena cava interposition graft.
Figure 2 illustrates the scanning electron microscopy of the PGA-P(CL/LA) scaffold. The internal diameter was approximately 1 mm and the wall thickness was approximately 0.17 mm. Total porosity was 78.5% and mean pore sizes were 45.4 ± 17.6 µm.
The gross image of a TEVG interposed into the IVC of C57BL/6 mice is shown in Figure 3. Right after implantation (A) and 2 weeks after implantation (B). (C) shows the gross image of a native IVC in comparison. It is evident that graft was filled with neotissue, however, it still kept the shape of the original graft. Our previous result showed that total graft degradation takes up to 12 weeks10.
The ultrasound B-mode and color Doppler image of a 2 weeks implanted TEVG Figures 4A-B to show the patency of the implanted grafts. A native IVC image was added for comparison (Figure 4, C). Cell seeding improved patency of the graft significantly, patencies at 2 weeks for unseeded and seeded grafts are 65% and 91%, respectively (p<0.05, Fisher's exact test).
Figure 5 shows the cell infiltration and extracellular matrix deposition in TEVG after 2 weeks of implantation. Explanted grafts stained with H&E staining showed neotissue formation throughout the graft and remainders of scaffold material (A). Harts and Masson's trichrome stain showed deposition of Elastin in the intimal layer (B) and collagen in the medial layer (C). Endothelial cell lining was observed throughout the intimal layer (D) and confluent smooth muscle cells were present throughout the TEVG, especially below the EC lining (E) as shown by CD31 and αSMA staining, respectively. By the second week after implantation, macrophage infiltration was evident, as indicated with F4/80 staining (F).

Figure 1. Schematic of TEVG implantation. Bone marrow mono nuclear cells were harvested from a donor mouse, seeded onto a biodegradable scaffold, and then implanted as an IVC interposition graft to a recipient mouse.

Figure 2. Scanning electron microscopy images of a scaffold. The average outer diameter was 1.45 mm, luminal diameter was 1.1 mm, and length was 3 mm.

Figure 3. Implanted TEVG after 2 weeks and native IVC. A) Immediately after implantation, B) 2 weeks after implantation, and C) native IVC for comparison.
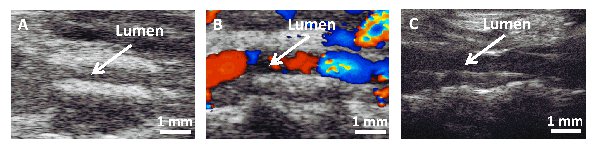
Figure 4. Ultrasound images 2 weeks and native IVC. A) B-Mode and B) color Doppler image of TEVG after 2 weeks. C) Native IVC for comparison.

Figure 5. Representative images of the 2 weeks post operative TEVG. A) H&E (inner lumen), B) Harts (pink = elastin), C) Massion's trichrome (blue = collagen), D) CD31 (brown = endothelial cell), E) αSMA (brown = smooth muscle cell), and F) F4/80 (brown = macrophage).
Subscription Required. Please recommend JoVE to your librarian.
Discussion
The mouse model of TEVG is a valuable tool to study cellular and molecular mechanisms of neotissue formation and the development of stenosis. The seeded BM-MNC was shown in both histological and SEM images of the seeded cells on the graft11. Cell seeding efficiency was also shown using a DNA assay7. Using this model system we showed that cell seeding reduces the incidence of the development of TEVG stenosis, which was the primary mode of failure in our human clinical trial3. The seeded cells rapidly disappeared from the TEVG, suggesting that they exert their effect via a paracrine mechanism8,12. It was also shown that neovessel formation is an immune-mediated regenerative process, and that the neovessel arises from ingrowth of the vascular cells (EC and SMC) from the neighboring vessel wall along the luminal surface of the scaffold. Moreover, normal macrophage infiltration is essential for vascular neotissue formation, but when this process is excessive it leads to the development of TEVG stenosis7,8. These results show the relevance of this mouse model to human disease. The microsurgical techniques can be adapted to other vascular applications including arterial grafts13 and artery to venous fistula.
We operate over 1,000 IVC interposition graft implantations each year and the mortality rate is less than 1%. For a successful graft implantation, there are several important points to mention.
First, injectable anesthesia is preferred for this operation because it is necessary to turn the mouse around several times during anastomosis. The effect of anesthesia usually lasts around an hour and the entire surgery time for a skilled micro surgeon is approximately 30-45 min, which provides enough time to complete the surgery. If the animal wakes up during the surgery, 0.05-0.1 ml of an anesthesia cocktail can be injected intramuscularly.
Second, most of the small aortic branches needed to be ligated to prevent excess blood loss while separating the IVC and the aorta from the abdominal area. Also keep in mind not to injure the IVC when it is separated from the aorta.
Third, when the graft was sutured, the first two sutures on both sides are the most important steps of the entire process. When they are not placed carefully, it is difficult to separate the front and back layers of the IVC. If the layers are not clearly identified, there is a higher chance to accidentally suture them together. While suturing the IVC to the graft, it is important not to over stretch the IVC, which can cause tearing. Also make sure not to cut off too much IVC to replace the same length of the graft. Usually approximately 1 to 2 mm of IVC was removed to replace a 3 to 5 mm graft due to the longitudinal tension of the IVC. If the same length of the IVC is removed, it makes anastomosis extremely challenging.
Fourth, there are significant strain to strain variations with respect to TEVG formation and stenosis development. For example, unseeded C57BL/6 mice (wild type) showed a much higher rate of stenosis than SCID/bg mice (immunodeficient mice)8,12. In addition, from our experience, SCID/bg mice are much easier to operate in comparison to C57BL/6 mice due to its stiffer IVC wall14. Keep that in mind when operating on a new strain of mice.
The graft manufacturing, BM-MNC harvesting, and cell seeding processes are straight forward, there are only a couple of processes to be cognizant of. First, for the graft manufacturing, it is very important not to 'bunch' the felt while push it using the 18 G blunt needle. We have tried different methods to push the felt down to the distal end of the pipet tips. Pushing the graft with a blunt needle showed the best outcome, by far. The inner diameter of the blunt needle is slightly bigger than the outer diameter of the needle that the felt is wrapped around. Therefore, the smaller needle can snugly slide into the bigger blunt needle and the felt can subsequently be pushed down with equal amounts of force all around the circumference of the felt, which prevents bunching of the felt. We previously observed that bunched grafts showed lower patency at 2 weeks. Also, it is critical to remove all bubbles because bubbles will cause holes on the scaffold surface and blood will leak through the hole after implantation. Second, when the bones are separated for BM-MNC harvesting, make sure to clean the bone as much as possible so they do not include too much muscle or hair. If necessary, it is recommended to use a tissue strainer before the density centrifugation process.
This model of TEVG worked well on venous circulation, which is a low pressure and high flow system. However, in arterial circulation, a high pressure and high flow system, we observed high rates of rupture with PGA grafts due to its fast degradation characteristic. Which means the graft loses its structural strength before the neotissue gains enough strength to resist the arterial pressure. We have used polylactic acid (PLA) grafts previously, but showed high prevalence of aneurysm formation. Also it takes more than 2 years to completely degrade PLA in vivo, which is more than the life span of mice. For a future application in arterial circulation graft manufacturing techniques, for example, an electrospun graft is currently under development.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported, in part, by a grant from the NIH (RO1 HL098228) to CKB.
Materials
| Name | Company | Catalog Number | Comments |
| Polyglycolic acid (PGA) felt | Biomedical Structures | Custome ordered | |
| Pipet tip, 0.1-10 μl | Fisher Sientific | 02-707-456 | |
| Lyophilizer | Labconco | 7070020 | |
| RPMI medium 1604 | Gibco | 11875-093 | |
| Petri dish | BD | 353003 | |
| 24-well plate | Corning | 3526 | |
| 15 cc tube | BD | 352096 | |
| Ficoll | Sigma | 10831-100ml | Also called 'Histopaque' |
| DPBS | Gibco | 14190-144 | |
| Littauer bone cutter 4.5" Straight | Roboz | RS-8480 | For BM harvesting |
| Forceps 4.5" | Roboz | RS-8120 | For BM harvesting |
| Scissors 4.5" | Roboz | RS-5912 | For BM harvesting |
| Microscope | Leica | M80 | |
| C57BL/6J (H-2b), Female | Jackson Laboratories | 664 | 8-12 weeks |
| Ketamine hydrochloride injection | Hospira Inc. | NDC 0409-2053 | |
| Xylazine sterile solution | Akorn Inc. | NADA# 139-236 | |
| Ketoprofen | Fort Dodge Animal Health | NDC 0856-4396-01 | |
| Ibuprofen | PrecisionDose | NDC 68094-494-59 | |
| Heparin sodium | Sagent Pharmaceticals | NDC 25021-400 | |
| Saline solution (sterile 0.9% sodium chloride) | Hospira Inc. | NDC 0409-0138-22 | |
| 0.9% Sodium chloride injection | Hospira Inc. | NDC 0409-4888-10 | |
| Petrolatum ophthalmic ointment | Dechra Veterinary Products | NDC 17033-211-38 | |
| Iodine prep pads | Triad Disposables, Inc. | NDC 50730-3201-1 | |
| Alcohol prep pads | McKesson Corp. | NDC 68599-5805-1 | |
| Cotton tipped applicators | Fisher Scientific | 23-400-118 | |
| Fine scissor | FST | 14028-10 | |
| Micro-adson forcep | FST | 11018-12 | |
| Clamp applying forcep | FST | 00072-14 | |
| S&T Vascular clamp | FST | 00396-01 | |
| Spring scissors | FST | 15008-08 | |
| Colibri retractors | FST | 17000-04 | |
| Dumont #5 forcep | FST | 11251-20 | |
| Dumont #7 - fine forceps | FST | 11274-20 | |
| Dumont #5/45 forceps | FST | 11251-35 | |
| Tish needle holder/forceps | Micrins | MI1540 | |
| Black polyamide monofilament suture, 10-0 | AROSurgical Instruments Corporation | TI638402 | For suturing the graft |
| Black polyamide monofilament suture, 6-0 | AROSurgical Instruments | SN-1956 | For musculature and skin closure |
| Non-woven sponges | McKesson Corp. | 94442000 | |
| Absorbable hemostat | Ethicon | 1961 | |
| 1 ml Syringe | BD | 309659 | |
| 3 ml Syringe | BD | 309657 | |
| 10 ml Syringe | BD | 309604 | |
| 18 G 1.5 in, Needle | BD | 305190 | |
| 25 G 1 in, Needle | BD | 305125 | |
| 30 G 1 in, Needle | BD | 305106 | |
| Warm water recirculator | Gaymar | TP-700 | |
| Warming pad | Gaymar | TP-22G | |
| Trimmer | Wahl | 9854-500 |
References
- Heart Association, A. merican Heart Disease and Stroke Statistics—2012 Update. Circulation. 125, (2012).
- Shinoka, T., et al. Creation Of Viable Pulmonary Artery Autografts Through Tissue Engineering. The Journal of Thoracic and Cardiovascular Surgery. 115, 536-546 (1998).
- Hibino, N., et al. Late-term results of tissue-engineered vascular grafts in humans. The Journal of Thoracic and Cardiovascular Surgery. 139, 431-436 (2010).
- Shin'oka, T., et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. The Journal of Thoracic and Cardiovascular Surgery. 129, 1330-1338 (2005).
- Brennan, M. P., et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 248, 370-377 (2008).
- Roh, J. D., et al. Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. Journal of Pediatric Surgery. 42, 198-202 (2007).
- Hibino, N., et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. The FASEB Journal. 25, 2731-2739 (2011).
- Hibino, N., et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. The FASEB Journal. 25, 4253-4263 (2011).
- Naito, Y., et al. Characterization of the Natural History of Extracellular Matrix Production in Tissue-Engineered Vascular Grafts during Neovessel Formation. Cells Tissues Organs. 195, 60-72 (2012).
- Naito, Y., et al. Beyond Burst Pressure: Initial Evaluation of the Natural History of the Biaxial Mechanical Properties of Tissue Engineered Vascular Grafts in the Venous Circulation Using a Murine Model. Tissue Eng. Part A. 20, (2013).
- Mirensky, T. L., et al. Tissue-engineered vascular grafts: does cell seeding matter. Journal of Pediatric Surgery. 45, 1299-1305 (2010).
- Roh, J. D., et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proceedings of the National Academy of Sciences. 107, 4669-4674 (2010).
- Mirensky, T. L., et al. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. Journal of Pediatric Surgery. 44, 1127-1133 (2009).
- Lee, Y. U., Naito, Y., Kurobe, H., Breuer, C. K., Humphrey, J. D. Biaxial mechanical properties of the inferior vena cava in C57BL/6 and CB-17 SCID/bg mice. Journal of Biomechanics. 46, 2277-2282 (2013).



