1.8:
Measurement: Derived Units
In 1960, the General Conference of Weights and Measures proposed the International System of Units, or the SI system, for measurement units.
This system defines seven base units for length, mass, time, temperature, amount of substance, electric current, and luminous intensity. These are called the Standard Units.
Units of measurements which are a combination of one or more base units are known as the derived units. Two of the most commonly used derived units in chemistry are volume and density.
Volume is a measure of the space occupied by an object. The SI unit for volume is defined by the base unit of length, the meter. Thus, any unit of length, when cubed or raised to a power of three, becomes a unit of volume. For example, m3, cm3, and mm3, are all units of volume.
Density, on the other hand, is the ratio of the mass of a substance to its volume. Thus the units for density are defined by the base units of mass and length. The SI unit for density is kg/m3. Often, g/m3 is used for the densities of solids and liquids, and g/L, for gases.
Standard and derived measurements are very helpful in defining various properties of matter. These properties can be broadly divided into two groups — intensive and extensive.
When the property is independent of the amount of matter present, it is known as an intensive property. Temperature, density, boiling and melting point, odor, and hardness are examples of intensive properties. The density of tin, for example, is 7.3 g/cm3 — and this remains the same whether we have a gram or a kilogram of tin. Hence, a density of 7.3 g/cm3 of a substance can be used to identify it as tin. In fact, all intensive properties can be used to identify a substance, as these characteristics do not change with the amount of, or conditions of, the sample.
An extensive property, on the other hand, depends on the amount of matter present. Mass, volume, and length are all extensive properties.
Extensive properties change with the sample size or conditions. This makes them a poor tool for identifying substances. The mass of a sample, for example, will not be helpful in identifying it as tin.
1.8:
Measurement: Derived Units
The International System of Units or SI system, by international agreement, has fixed measurement units for seven fundamental properties: length, mass, time, temperature, electric current, amount of substance, and luminosity. These are called the SI base units.
Units of measurement derived from the mathematical combination of SI base units are called SI-derived units. For example, the ratio of the SI unit for distance (meter; m) and the SI unit for time (second; s) gives the SI-derived unit for speed (meter per second; m/s). Another common way of expressing the speed of an object is using miles per hour (miles/hour). Miles per hour is also considered a derived unit, even though the base units are not SI units. In general, any derived unit is a combination of other units.
Derived Units: Volume and Density
Volume is the measure of the amount of space occupied by an object. The unit of length defines the unit of volume.
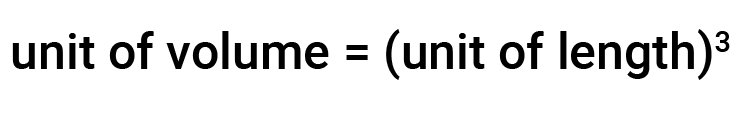
The SI-derived unit of volume is a cubic meter (m3), a cube with an edge length of exactly one meter. To dispense a cubic meter of water, we could build a cubic box with edge lengths of exactly one meter. This box would hold a cubic meter of water or any other substance.
Other common units of volume are the cubic decimeter (dm3) and the cubic centimeter (cm3). A cube with edge lengths of exactly one decimeter (or 10 cm) contains a volume of one cubic decimeter (1 dm3 or 1000 cm3). A liter (L) is the more common name for the cubic decimeter. One liter is equal to 1000 milliliters, and one milliliter is equal to 1 cubic centimeter.
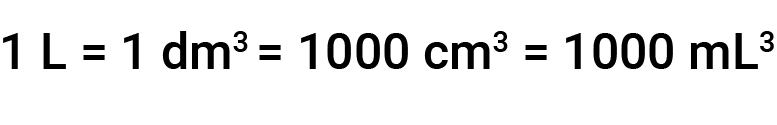
The density of a substance is the ratio of the mass of the substance to its volume
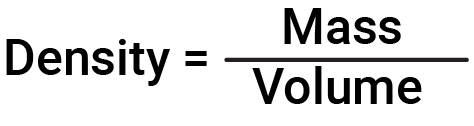
Thus, the units of density are defined by the units of mass and length (volume = length3). Since the SI unit of mass is the kilogram (kg) and volume is the cubic meter (m3), the SI-derived unit for density is the kilogram per cubic meter (kg/m3).
Another common unit, grams per cubic centimeter (g/cm3) is often used for the densities of solids and liquids, and grams per liter (g/L) for gases.
Extensive and Intensive Properties of Matter
A measurement unit expresses the magnitude of a physical quantity that is used to define a physical property of matter. Physical properties can be extensive or intensive. If the property depends on the amount of matter present, it is an extensive property. Extensive properties include mass, weight, and volume. For example, a liter of milk has a larger mass than a cup of milk. The value of an extensive property is directly proportional to the amount of matter in question. On the contrary, if the property does not depend on the amount of matter present, it is an intensive property. Temperature is an example of an intensive property. If one liter of milk and one cup of milk are each at 20 °C, their temperature remains at 20 °C when they are combined. Consider another example to understand the distinct but related properties of heat and temperature. A drop of hot cooking oil splattered on your arm causes brief, minor discomfort, whereas a pot of hot oil yields severe burns. Both the drop and the pot of oil are at the same temperature (an intensive property), but the pot of oil contains much more heat (extensive property).
This text is adapted from Openstax, Chemistry 2e, Section 1.3: Physical and Chemical Properties and Openstax, Chemistry 2e, Section 1.4: Measurements.
