7.1:
The Wave Nature of Light
The light from the sun, the microwaves used to cook food, and the radio waves emitted by wi-fi routers are all examples of electromagnetic radiation.
Electromagnetic radiation is the transmission of the energy that comes from the motion of electrically charged particles.
This movement creates perpendicular oscillating electric and magnetic fields propagating through space in the form of waves.
Like all waves, electromagnetic waves are characterized by their amplitude, wavelength, and frequency.
The peak amplitude is the distance from the midline to the peak or trough of a wave. It determines the intensity of the wave. For example, the amplitude of visible light is related to its brightness; the larger the amplitude, the brighter, or more intense, the light.
The wavelength is the distance between identical points on adjacent waves, such as successive peaks or troughs; it is symbolized by the Greek letter lambda. Electromagnetic radiation can be categorized by wavelength, which can range from kilometers to picometers.
For example, the wavelength range of visible light is about 400 to 750 nanometers, which correspond to violet and red light, respectively.
The frequency, which is symbolized by the Greek letter nu, is the number of wave cycles that pass through a reference point in one second and is measured in hertz, or cycles per second. The wavelength is the width of one cycle.
The speed of a wave is the product of its frequency and wavelength. Hence, the frequency of a wave is directly proportional to the speed at which the wave is traveling. However, it is inversely proportional to the wavelength of the wave.
Therefore, waves with long wavelengths, such as radio waves, have low frequencies. These waves are less energetic than waves with high frequencies and short wavelengths, such as gamma rays.
7.1:
The Wave Nature of Light
The nature of light has been a subject of inquiry since antiquity. In the seventeenth century, Isaac Newton performed experiments with lenses and prisms and was able to demonstrate that white light consists of the individual colors of the rainbow combined together. Newton explained his optics findings in terms of a "corpuscular" view of light, in which light was composed of streams of extremely tiny particles traveling at high speeds according to Newton's laws of motion.
Others in the seventeenth century, such as Christiaan Huygens, had shown that optical phenomena such as reflection and refraction could be equally well explained in terms of light as waves traveling at high speed through a medium called "luminiferous aether" that was thought to permeate all space. Early in the nineteenth century, Thomas Young demonstrated that light passing through narrow, closely spaced slits produced interference patterns that could not be explained in terms of Newtonian particles but could be easily explained in terms of waves. Later in the nineteenth century, after James Clerk Maxwell developed his theory of electromagnetic radiation and showed that light was the visible part of a vast spectrum of electromagnetic waves, the particle view of light became thoroughly discredited.
By the end of the nineteenth century, scientists viewed the physical universe as roughly comprising two separate domains: matter composed of particles moving according to Newton's laws of motion, and electromagnetic radiation consisting of waves governed by Maxwell's equations. Today, these domains are referred to as classical mechanics and classical electrodynamics (or classical electromagnetism). Although there were a few physical phenomena that could not be explained within this framework, scientists at that time were so confident of the overall soundness of this framework that they viewed these aberrations as puzzling paradoxes that would ultimately be resolved somehow within this framework. These paradoxes led to a contemporary framework that intimately connects particles and waves at a fundamental level called wave-particle duality, which has superseded the classical view.
Visible light and other forms of electromagnetic radiation play important roles in chemistry since they can be used to infer the energies of electrons within atoms and molecules. Much of modern technology is based on electromagnetic radiation. For example, radio waves from a mobile phone, X-rays used by dentists, the energy used to cook food in your microwave, the radiant heat from red-hot objects, and the light from your television screen are forms of electromagnetic radiation that all exhibit wavelike behavior.
Waves
A wave is an oscillation or periodic movement that can transport energy from one point in space to another. Common examples of waves are all around us. Shaking the end of a rope transfers energy from your hand to the other end of the rope, dropping a pebble into a pond causes waves to ripple outward along the water's surface, and the expansion of air that accompanies a lightning strike generates sound waves (thunder) that can travel outward for several miles. In each of these cases, kinetic energy is transferred through matter (the rope, water, or air) while the matter remains essentially in place.
Waves need not be restricted to travel through matter. As Maxwell showed, electromagnetic waves consist of an electric field oscillating in step with a perpendicular magnetic field, both of which are perpendicular to the direction of travel. These waves can travel through a vacuum at a constant speed of 2.998 × 108 m/s, the speed of light (denoted by c).
All waves, including forms of electromagnetic radiation, are characterized by a wavelength (denoted by λ, the lowercase Greek letter lambda), a frequency (denoted by ν, the lowercase Greek letter nu), and an amplitude.
The wavelength is the distance between two consecutive peaks or troughs in a wave (measured in meters in the SI system). Electromagnetic waves have wavelengths that fall within an enormous range-wavelengths of kilometers (103 m) to picometers (10−12 m) have been observed. The frequency is the number of wave cycles that pass a specified point in space in a specified amount of time (in the SI system, this is measured in seconds). A cycle corresponds to one complete wavelength. The unit for frequency, expressed as cycles per second [s−1], is the hertz (Hz). Common multiples of this unit are megahertz (1 MHz = 1 × 106 Hz) and gigahertz (1 GHz = 1 × 109 Hz).
The amplitude corresponds to the magnitude of the wave's displacement, and this corresponds to one-half the height between the peaks and troughs. The amplitude is related to the intensity of the wave, which for light is the brightness, and for sound is the loudness. The product of a wave's wavelength (λ) and its frequency (ν), λν, is the speed of the wave. Thus, for electromagnetic radiation in a vacuum, speed is equal to the fundamental constant, c:
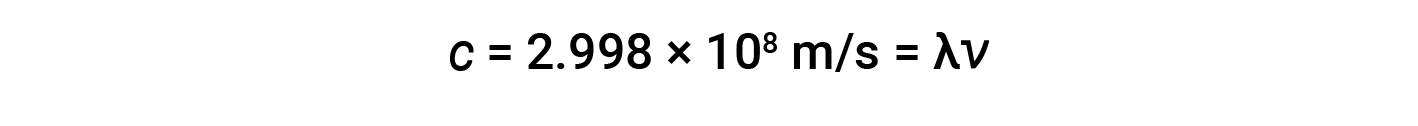
Wavelength and frequency are inversely proportional: As the wavelength increases, the frequency decreases. The electromagnetic spectrum is the range of all types of electromagnetic radiation.
This text is adapted from Openstax, Chemistry 2e, Section 6.1: Electromagnetic Energy.
