8.5:
Electron Affinity
When an electron is added to a gaseous atom, a change in energy is observed called electron affinity. Electron affinity measures the ease of gaining an electron by an atom.
For example, the electron affinity of chlorine is −348.6 kJ/mol. The negative sign indicates that it is an exothermic change.
Argon, however, has a positive electron affinity, indicating that formation of an argon anion requires energy to be supplied.
In general, the greater the attraction between an atom and an added electron, the more negative the electron affinity. Electron affinities, similar to ionization energies, show trends in the periodic table.
Moving down group 1, the atomic size increases as the electrons occupy higher principal quantum numbers. Incoming electrons, therefore, experience less nuclear attraction leading to less negative electron affinities.
However, there are exceptions. In halogens, chlorine has a more negative electron affinity value than fluorine. But why?
Fluorine is the smallest atom of the halogens and an incoming electron experiences a significant repulsion from the electrons already present.
In the chloride anion, however, the new electron is added into the third shell, occupying more space. This reduces the electron-electron repulsions, making it more attractive for an electron to be gained.
Generally, moving across a period, electron affinities become more negative. Halogens have the most negative electron affinities, as the incoming electron helps to achieve noble gas configurations.
In comparison, noble gases have a completely filled shell. The incoming electron has to be accommodated in the higher principal energy level, which is energetically unfavorable. Thus, electron affinities for these elements are positive.
Group 2 shows exceptions. The electron configuration indicates that the incoming electron needs to enter a higher-energy subshell. Thus, electron affinity values are either positive or less exothermic.
Interestingly, group 15 has less negative electron affinities than group 14. Compare phosphorus and silicon. Unlike silicon, phosphorus has a half-filled p-subshell and the incoming electron needs to be paired with an electron already residing in the p-orbital.
This would increase the electron-electron repulsions and is therefore an energetically unfavourable process, which is also reflected in the less negative electron affinity compared to silicon.
8.5:
Electron Affinity
The electron affinity (EA) is the energy change for adding an electron to a gaseous atom to form an anion (negative ion).
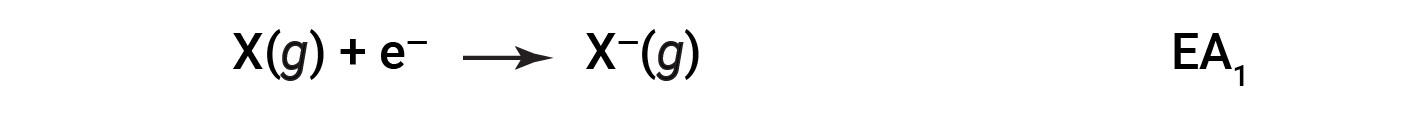
This process can be either endothermic or exothermic, depending on the element. Many of these elements have negative values of EA, which means that energy is released when the gaseous atom accepts an electron. However, for some elements, energy is required for the atom to become negatively charged, and the value of their EA is positive. Just as with ionization energy, subsequent EA values are associated with forming ions with more charge. The second EA is the energy associated with adding an electron to an anion to form a 2– ion, and so on.
As one might predict, it becomes easier to add an electron across a series of atoms as the effective nuclear charge of the atoms increases. As we go from left to right across a period, EAs tend to become more negative. The exceptions found among the elements of group 2 (2A), group 15 (5A), and group 18 (8A) can be understood based on the electronic structure of these groups. The noble gases, group 18 (8A), have a completely filled shell, and the incoming electron must be added to a higher n level, which is more difficult to do. Group 2 (2A) has a filled ns subshell, and so the next electron added goes into the higher energy np, so, again, the observed EA value is not as the trend would predict. Finally, group 15 (5A) has a half-filled np subshell, and the next electron must be paired with an existing np electron. In all of these cases, the initial relative stability of the electron configuration disrupts the trend in EA.
One might expect the atom at the top of each group to have the most negative EA; their first ionization potentials suggest that these atoms have the largest effective nuclear charges. However, as we move down a group, we see that the second element in the group most often has the most negative EA. This can be attributed to the small size of the n = 2 shell and the resulting large electron-electron repulsions. For example, chlorine, with an EA value of –348 kJ/mol, has the highest value of any element in the periodic table. The EA of fluorine is –322 kJ/mol. When we add an electron to a fluorine atom to form a fluoride anion (F–), we add an electron to the n = 2 shell. The electron is attracted to the nucleus, but there is also significant repulsion from the other electrons already present in this small valence shell. The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron-electron repulsions are reduced. The entering electron does not experience as much repulsion, and the chlorine atom accepts an additional electron more readily, resulting in a more negative EA.
This text is adapted from OpenStax Chemistry 2e, Section 6.5: Periodic Variations in Element Properties.
