10.3:
Predicting Molecular Geometry
VSEPR theory helps to determine electron-pair geometries and molecular geometries.
A series of steps is used to predict the geometry and bond angles of molecules, such as phosphorus trichloride.
The first step is to draw the Lewis structure of the molecule.
Next, count the total number of electron groups on the central atom. Around phosphorus, there are four electron groups: three bonding pairs and one lone pair.
Now determine the electron-pair geometry. The electron pair geometry is tetrahedral. However, because of the lone pair, the molecular geometry is trigonal pyramidal. The lone pair reduces the bond angle to less than 109.5°.
The same protocol is used to predict the electron-pair geometry and molecular structure for carbon dioxide.
The Lewis structure of carbon dioxide shows the two-electron groups around the carbon atom—as each double bond counts as one electron group. The two-electron groups orient themselves on opposite sides of the central carbon atom with a bond angle of 180°. The electron-pair and molecular geometries are identical because there are no lone pairs on the central atom, and carbon dioxide molecules are linear.
The Lewis structure of tellurium tetrachloride has five electron groups around the tellurium atom: four bonding pairs and one lone pair. The electron groups have a trigonal bipyramidal geometry. The lone pair occupies one of the equatorial positions, and the molecule is seesaw-shaped.
These steps can again be used to determine the electron-pair geometry and molecular structure of the iodine tetrachloride anion.
The Lewis structure has six electron groups around the iodine atom: four bonding pairs and two lone pairs. The electron groups have an octahedral arrangement. The bonding pairs stay in one plane and the lone pairs are placed on either side of this plane, minimizing the repulsion. The molecular geometry is square planar.
10.3:
Predicting Molecular Geometry
VSEPR Theory for Determination of Electron Pair Geometries
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures:
- Write the Lewis structure of the molecule or polyatomic ion.
- Count the number of electron groups (lone pairs and bonds) around the central atom. A single, double, or triple bond counts as one region of electron density.
- Identify the electron-pair geometry based on the number of electron groups: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (As depicted in Figure 1, first column).
- Use the number of lone pairs to determine the molecular structure. If more than one arrangement of lone pairs and chemical bonds is possible, choose the one that will minimize repulsions, remembering that lone pairs occupy more space than multiple bonds, which occupy more space than single bonds. In trigonal bipyramidal arrangements, repulsion is minimized when every lone pair is in an equatorial position. In an octahedral arrangement with two lone pairs, repulsion is minimized when the lone pairs are on opposite sides of the central atom.
The molecular structures are identical to the electron-pair geometries when there are no lone pairs present. For a particular number of electron pairs, the molecular structures for one or more lone pairs are determined based on modifications of the corresponding electron-pair geometry.
Predicting Molecular Structures using VSEPR Theory
The following examples illustrate the use of VSEPR theory to predict the molecular structures.
Let’s see how to determine the electron-pair geometry and molecular structure of CO2 and BCl3.
We write the Lewis structure of CO2 as:
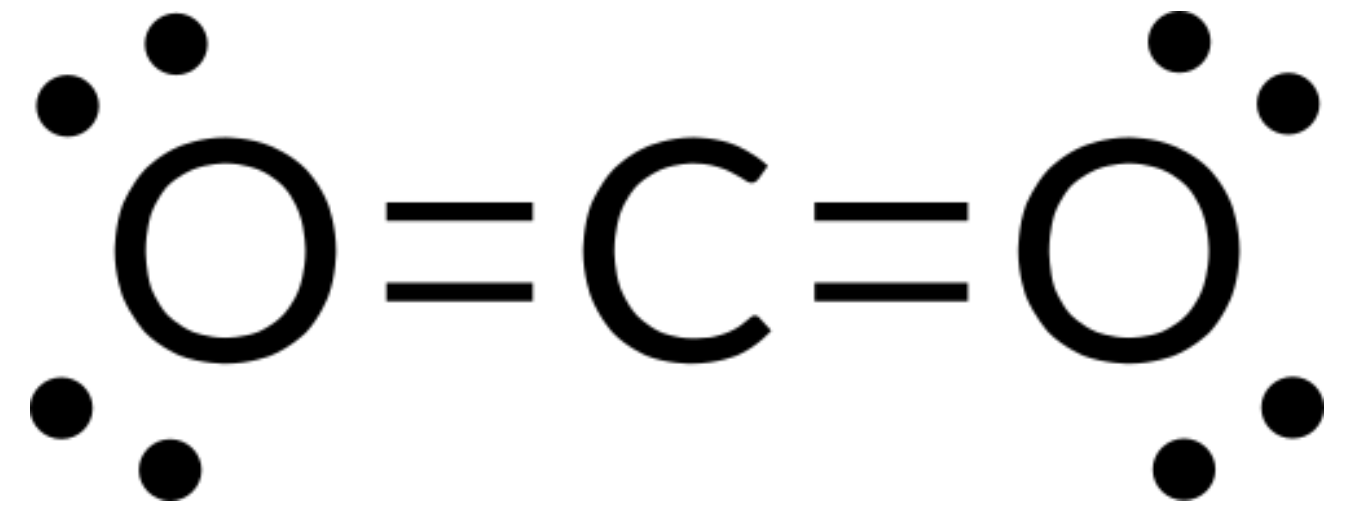
This shows us two double bonds around the carbon atom—each double bond counts as one electron group, and there are no lone pairs on the carbon atom. Using VSEPR theory, we predict that the two electron groups arrange themselves on opposite sides of the central atom with a bond angle of 180°. The electron-pair geometry and molecular structure are identical, and CO2 molecules are linear.
To predict the electron-pair geometry and molecular structure of a TeCl4 molecule, the first step is to write the Lewis structure of TeCl4. It indicates five electron groups around the Te atom: one lone pair and four bonding pairs:
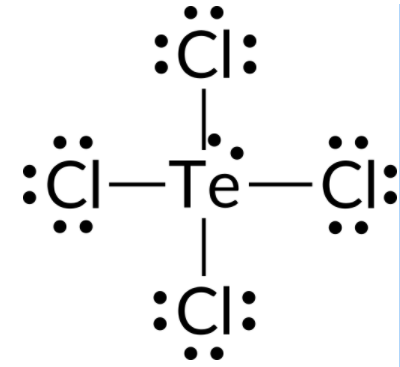
We expect these five electron groups to adopt a trigonal bipyramidal electron-pair geometry. To minimize lone pair repulsions, the lone pair occupies one of the equatorial positions. The molecular structure is that of a seesaw.
This text has been adapted from Openstax, Chemistry 2e, Section 7.6: Molecular Structure and Polarity.
