14.5:
Reaction Quotient
The equilibrium constant expression is written as the molar concentrations of the products, C and D, over the reactants, A and B, at equilibrium, each raised to their respective stoichiometric coefficients. When solved, the expression is equal to the equilibrium constant, Kc.
An expression in the same form can also be written for the reactants and products at any concentration, and the calculated quantity is known as the reaction quotient, Qc.
Like Qc, the Qp expression can be written for gaseous reactions using partial pressures.
While K remains constant at a specific temperature irrespective of concentration, the value of Q changes as the reaction proceeds towards the products or the reactants.
The reaction quotient can be used to determine the direction a reaction will proceed in order to reach equilibrium.
At the start of a given reaction, if the concentration of the products is zero, the reaction quotient is zero.
Whenever the concentration of the reactants in the denominator is high, such that Q is smaller than K, the reaction will move to the right to synthesize more products until the system reaches equilibrium.
If the concentration of the reactants is zero, the reaction quotient is infinite.
Whenever the concentration of the products in the numerator is high, such that Q is larger than K, the reaction will move to the left to produce more reactants.
If Q is equal to K, the system is at equilibrium, and the rate of the forward and reverse reactions are equal.
Consider the given reaction with an equilibrium constant 50. If the reaction mixture contains 0.20 molar hydrogen, 0.20 molar iodine, and 1.7 molar hydrogen iodide, the direction of the reaction shown can be determined by calculating Q.
Substituting the given concentrations into the expression, Q equals 72, which is greater than K. Therefore, the reaction will shift towards the left.
14.5:
Reaction Quotient
The status of a reversible reaction is conveniently assessed by evaluating its reaction quotient (Q). For a reversible reaction described by m A + n B ⇌ x C + y D, the reaction quotient is derived directly from the stoichiometry of the balanced equation as
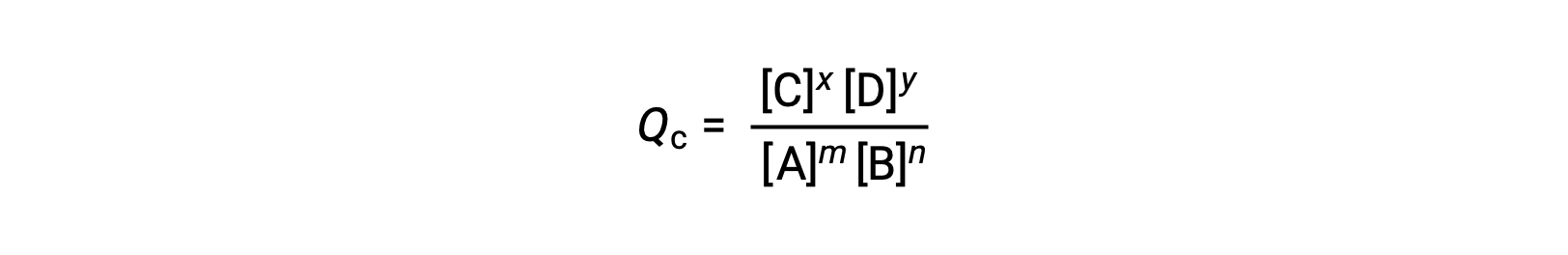
where the subscript c denotes the use of molar concentrations in the expression. If the reactants and products are gaseous, a reaction quotient may be similarly derived using partial pressures:
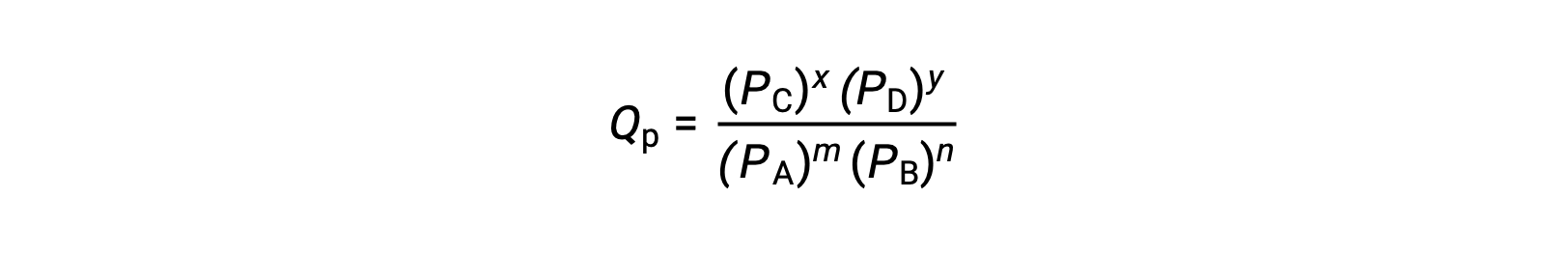
Note that the reaction quotient equations above are a simplification of more rigorous expressions that use relative values for concentrations and pressures rather than absolute values. These relative concentration and pressure values are dimensionless (they have no units); consequently, so are the reaction quotients.
The numerical value of Q varies as a reaction proceeds towards equilibrium; therefore, it can serve as a useful indicator of the reaction’s status. To illustrate this point, consider the oxidation of sulfur dioxide:
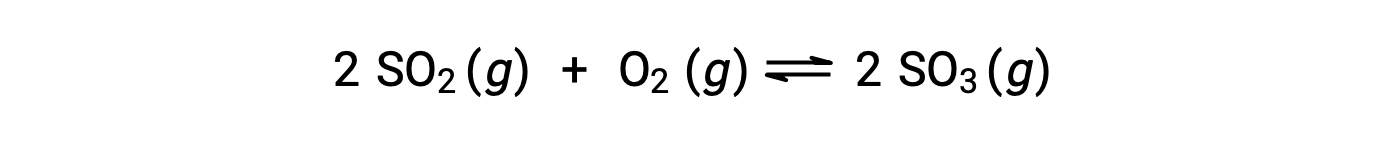
Two different experimental scenarios are possible here, one in which this reaction is initiated with a mixture of reactants only, SO2 and O2, and another that begins with only product, SO3. For the reaction that begins with a mixture of reactants only, Q is initially equal to zero:
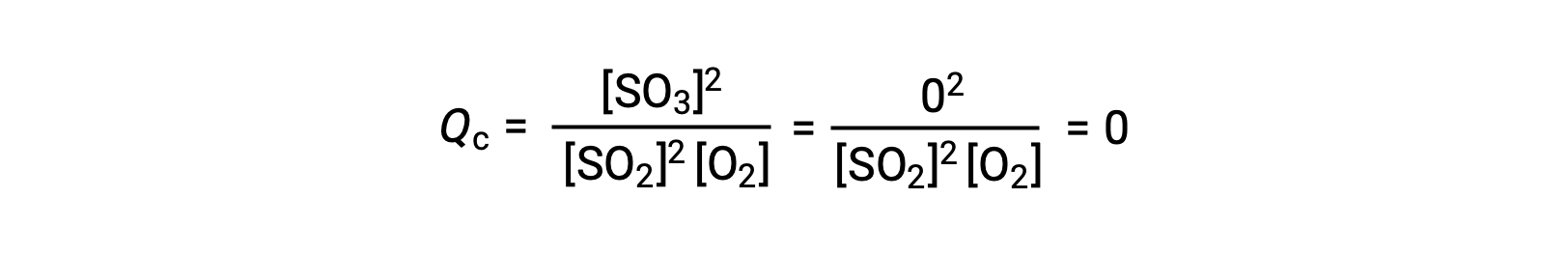
As the reaction proceeds toward equilibrium in the forward direction, reactant concentrations decrease (as does the denominator of Qc), product concentration increases (as does the numerator of Qc), and the reaction quotient consequently increases. When equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of Qc.
If the reaction begins with only product present, the value of Qc is initially undefined (immeasurably large, or infinite):
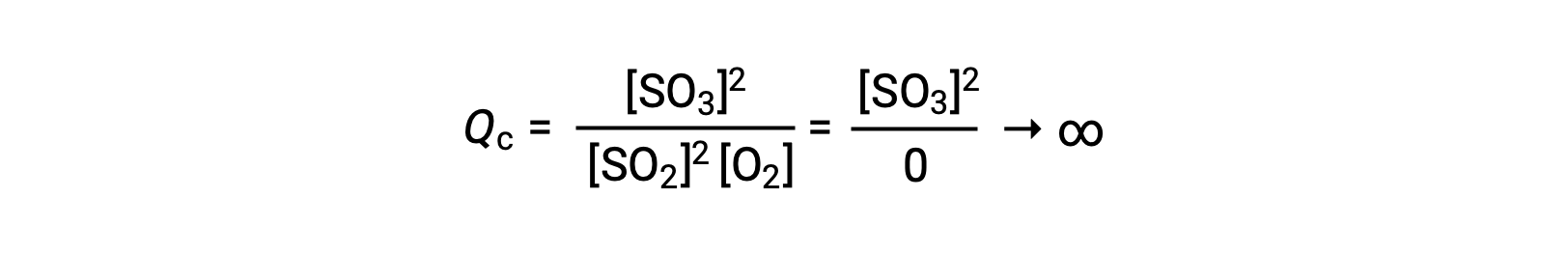
In this case, the reaction proceeds toward equilibrium in the reverse direction. The product concentration and the numerator of Qc decrease with time, the reactant concentrations and the denominator of Qc increase, and the reaction quotient consequently decreases until it becomes constant at equilibrium. The constant value of Q exhibited by a system at equilibrium is called the equilibrium constant, K:
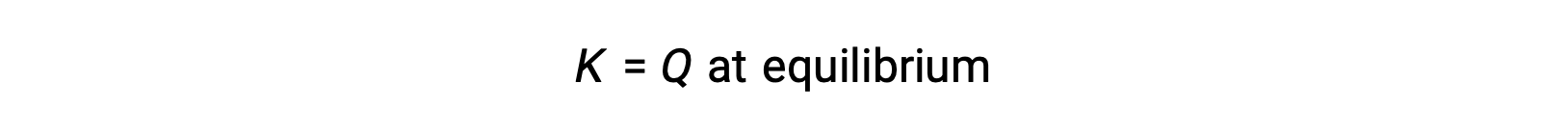
Evaluating a Reaction Quotient
Gaseous nitrogen dioxide forms dinitrogen tetroxide according to this equation:
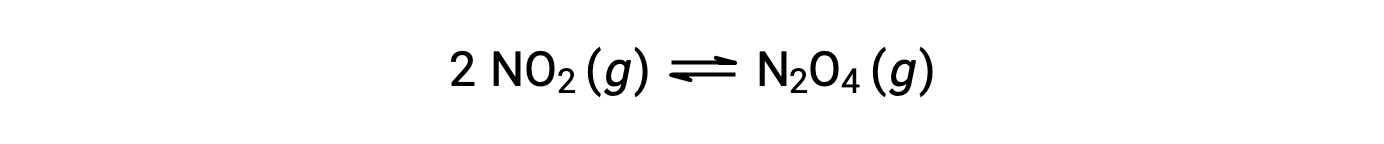
When 0.10 mol NO2 is added to a 1.0-L flask at 25 °C, the concentration changes so that at equilibrium, [NO2] = 0.016 M and [N2O4] = 0.042 M. Before any product is formed, [NO2] = 0.10 M and [N2O4] = 0 M. Thus,
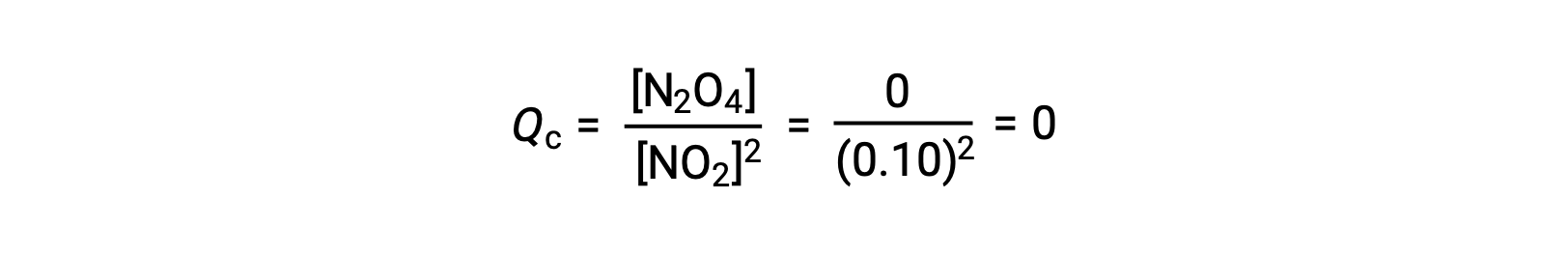
At equilibrium,
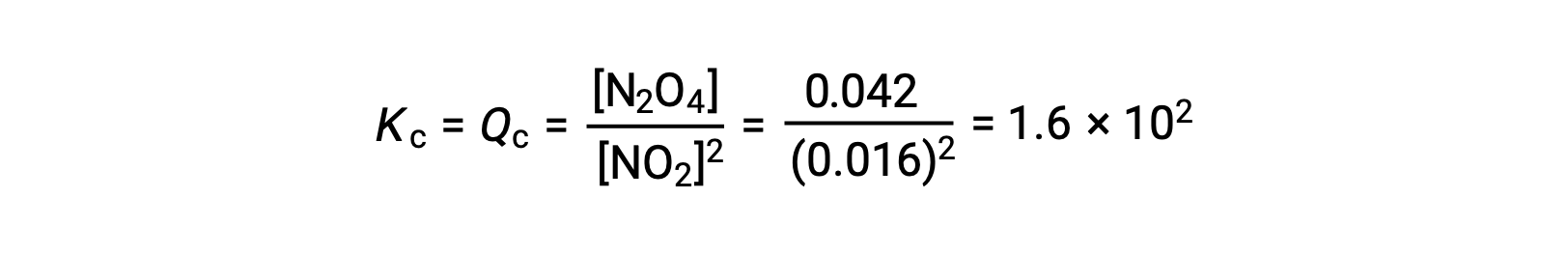
This text has been adapted from Openstax, Chemistry 2e, Section 13.2 Equilibrium Constants.
Suggested Reading
- Lederer, Robert. "The Reaction Quotient Is Unnecessary To Solve Equilibrium Problems: No Problems with Q." Journal of Chemical Education 82, no. 8 (2005): 1149. https://pubs.acs.org/doi/pdf/10.1021/ed082p1149.2
