4.4:
The Equilibrium Binding Constant and Binding Strength
In the cell, proteins randomly collide with other molecules, such as other proteins, nucleic acids, and small-molecule ligands.
If the binding is non-specific, few non-covalent interactions between the molecules result in a brief association.
If a specific ligand binds to the protein, it forms extensive non-covalent interactions along complementary surfaces. Such complexes are stable, staying bound for a long time, before dissociating.
The strength of a binding interaction is reported in terms of its equilibrium constant, Kb, also called the binding or association constant.
Kb can be calculated from the ratio of the concentration of protein-ligand complex over the concentrations of unbound protein and ligand found at equilibrium.
Given its relation with the free energy change due to binding, a large Kb means a large decrease in delta G, indicating strong affinity between protein and ligand.
Two competing processes are important for protein-ligand binding: the association of a protein and a ligand to form a complex and the dissociation of the complex into the reactants.
The association constant, kon, is a measure of the number of binding events per second between a protein and its ligand; it can be used to calculate the rate of ligand binding to the protein at a given concentration.
Conversely, koff, is a measure of the number of dissociation events; it can be used to calculate how quickly the complex comes apart.
When the rate of association equals the rate of dissociation, an equilibrium is reached, where the net concentrations of products and reactants remain constant.
Thus, at equilibrium, kon times the product of the equilibrium concentrations of the protein and the ligand equals koff times the equilibrium concentration of the protein-ligand complex.
Rearranging this expression also shows that the ratio of kon to koff equals Kb at equilibrium.
4.4:
The Equilibrium Binding Constant and Binding Strength
The equilibrium binding constant (Kb) quantifies the strength of a protein-ligand interaction. Kb can be calculated as follows when the reaction is at equilibrium:
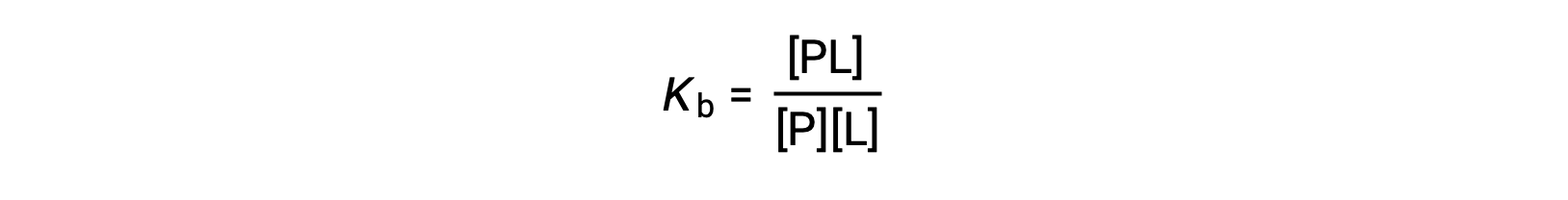
where P and L are the unbound protein and ligand, respectively, and PL is the protein-ligand complex.
As the amount of bound ligand is also related to the rate of ligand binding, experiments can also determine Kb by examining the rates of protein-ligand association (kon) and dissociation (koff) using the following ratio:
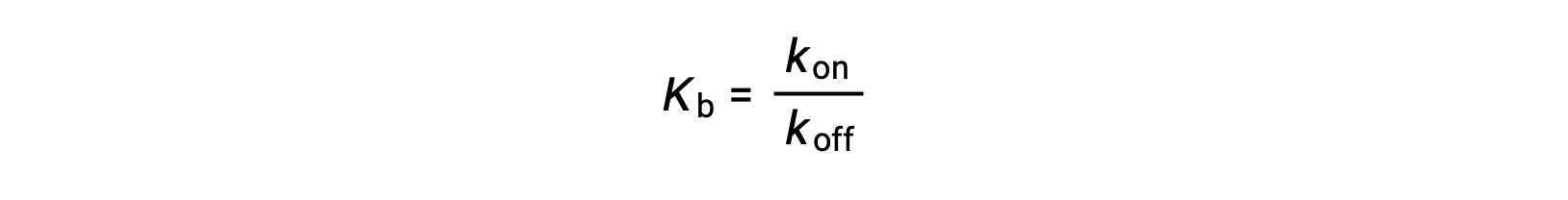
Thus, two categories of binding assays are used to determine the equilibrium binding constant – those that measure equilibrium concentrations and those that measure a reaction’s kinetics. In case, the reaction must be at equilibrium at the time of measurement.
The method of determining the equilibrium concentrations depends on the desired sensitivity and ease of signal detection. For these reasons, spectroscopic assays are most widely used. In these experiments, the reaction produces an absorbance change of a reactant or a product at a given wavelength, detectable by a UV-Vis spectrophotometer. Alternatively, the reactant or product can be tagged with a fluorescent probe or may contain an intrinsic fluorophore. Then, the reaction progress can be measured from the change in fluorescence. These assays are performed by varying one reactant’s concentrations while the rest of the experiment is held constant. The results can then be graphed and analyzed with various curve fitting methods.
Interactions between proteins and ligands are also studied using a variety of biochemical and spectroscopic techniques. Structural analysis, using X-ray crystallography and NMR spectroscopy, aids in predicting protein-ligand interactions through molecular simulations. Theoretical and computational approaches, such as protein-ligand docking studies, are used extensively to characterize the position and interactions of small molecule ligands, including drug candidates. Computer-aided drug design is a fast and low-cost alternative to accelerate the pace of conventional trial and error drug testing.
Suggested Reading
- Pollard, Thomas D. "A guide to simple and informative binding assays." Molecular biology of the cell 21, no. 23 (2010): 4061-4067.
- Hulme, Edward C., and Mike A. Trevethick. "Ligand binding assays at equilibrium: validation and interpretation." British journal of pharmacology 161, no. 6 (2010): 1219-1237.
- Medina-Franco, José L., Oscar Méndez-Lucio, and Karina Martinez-Mayorga. "The interplay between molecular modeling and chemoinformatics to characterize protein–ligand and protein–protein interactions landscapes for drug discovery." In Advances in protein chemistry and structural biology, vol. 96, pp. 1-37. Academic Press, 2014.
