Overview
Source: Laboratory of Dr. Nicholas Leadbeater — University of Connecticut
Distillation is perhaps the most common laboratory technique employed by chemists for the purification of organic liquids. Compounds in a mixture with different boiling points separate into individual components when the mixture is carefully distilled. The two main types of distillation are "simple distillation" and "fractional distillation", and both are widely used in organic chemistry laboratories.
Simple distillation is used when the liquid is (a) relatively pure (containing no more than 10% liquid contaminants), (b) has a non-volatile component, such as a solid contaminant, or (c) is mixed with another liquid with a boiling point that differs by at least 25 °C. Fractional distillation is used when separating mixtures of liquids whose boiling points are more similar (separated by less than 25 °C).
This video will detail the fractional distillation of a mixture of two common organic solvents, cyclohexane and toluene.
Principles
To separate two or more liquids by simple distillation, they are first heated in a flask. The more volatile liquid (the liquid with the lower boiling point) will typically evaporate first, and the vapor will pass into a condensing column, where it can revert into a liquid (condense) on the cool glass walls and then drip into a collection vessel (Figure 1).

Figure 1. Apparatus for simple distillation.
In fractional distillation, a mixture of liquids is boiled and the resulting vapors travel up a glass tube called a "fractionating column" and separate. The fractionating column is placed between the flask containing the mixture and the "Y" adaptor, and is usually filled with glass or plastic beads, which improves the separation between the liquids being distilled. Fractional distillation leads to a better separation than simple distillation because the glass beads in the fractionating column provide 'theoretical plates' on which the vapors can condense, reevaporate, and then recondense, essentially distilling the mixture over and over. One theoretical plate is equivalent to one vaporization-condensation cycle, which is equivalent to one simple distillation. The more volatile liquids will gradually move towards the top of the fractionating column, while lower boiling liquids will stay towards the bottom, giving a better separation between the liquids. The vapor eventually reaches the condenser, where it is cooled and then drips into the collection vessel.
A boiling point composition curve (Figure 2) can be used to predict the number of theoretical plates needed to achieve a desired separation. Data for the curve is obtained by taking mixtures of varying composition, heating them to the boiling point, recording the temperature, and analyzing the composition of the vapor above each mixture. The lower curve represents the liquid composition and the upper curve represents the vapor composition. In the case of this experiment, the starting mixture was 50% cyclohexane and 50% toluene. By starting at the X-axis at the 50:50 point, drawing a line straight up to the liquid curve, then a straight horizontal line over to the vapor curve and then back down to the X-axis, it can be seen that during the simple distillation of a 50% cyclohexane : 50% toluene mixture, the first drop of distillate would be comprised of approximately 80% cyclohexane and 20% toluene.

Figure 2. Boiling point-composition curve for cyclohexane and toluene showing one simple distillation.
Fractional distillation is basically a number of simple distillations performed in sequence, the number being determined by the number of theoretical plates. It is possible to use the boiling point composition curve to determine the number of theoretical plates required to obtain a certain degree of separation (Figure 3). Starting with a mixture that is 50% cyclohexane and 50% toluene, a fractionating column with an efficiency equal to two theoretical plates would result in distillate that is 95% pure cyclohexane. A third theoretical plate would result in distillate that is about 99% pure cyclohexane. However, in practice, the situation is not as simple. As the distillation proceeds and the mixture in the distilling flask becomes increasingly enriched in toluene — essentially, resulting in a new starting mixture that is further to the right on the liquid composition curve. In order to obtain pure cyclohexane, more theoretical plates are needed, but the efficiency of the fractionating column is fixed, and so a point is reached at which it can no longer provide the required degree of separation, resulting in distillate that contains more contaminating traces of toluene.

Figure 3. Boiling point-composition curve for cyclohexane and toluene showing three theoretical plates.
A distillation curve, which plots temperature vs. volume of distillate, is shown in Figure 4. Distillation initially occurs at about 82 °C, the boiling point of cyclohexane. The relatively stable temperature shows that nearly pure material is distilling during this time. The temperature then rises and reaches another plateau around 110 °C, the boiling point of toluene. Between the two plateaus the temperature rises gradually, not instantly. Recall that as the distillation proceeds and the cyclohexane is removed, more theoretical plates are required to obtain the desired purity. At some point the fractionating column is at its maximum number of plates and can no longer effectively separate the mixture, meaning that there will be distillate containing both the cyclohexane and toluene components. Once all the cyclohexane has been distilled, however, pure toluene begins distilling as evidenced by the second temperature plateau. To obtain the optimal distillation, the distillation rate must be constant and relatively slow.

Figure 4. Fractional distillation curve for cyclohexane and toluene.
Subscription Required. Please recommend JoVE to your librarian.
Procedure
1. Set-up of Fractional Distillation Apparatus
| Fractional Distillation Apparatus | Quantity |
| Retort stands (ring stands) | 2 |
| Screw jack | 1 |
| Clamps | 4 |
| Keck clamps | 4 |
| Magnetic stir bar | 1 |
| Fractionating column | 1 |
| “Y” adaptor | 1 |
| Thermometer & Thermometer Adaptor | 1 |
| 100-mL round bottom flask | 1 |
| Condenser with water inlet and outlet tubes | 1 |
| Collection adaptor | 1 |
| 25-mL graduated cylinders | 3 |
| Heating mantle | 1 |
| Stir plate | 1 |
Table 1. Fractional distillation apparatus components.
Note: all glassware, with the exception of the graduated cylinders, should have ground-glass joints
- Gather all of the equipment needed to assemble the fractional distillation apparatus (Table 1) and place them in a fume hood.
- Place the heating mantle and stir plate at the foot of the retort stand, sitting on top of a screw jack.
- Using the screw jack, elevate the heating mantle to a height of 20 cm.
- Attach a clamp on the retort stand. Secure the round-bottom flask to the retort stand using the clamp so that the flask sits snuggly in the heating mantle.
- Remove the screw jack and the heating mantle, putting them aside for later.
- Add a magnetic stir bar to the round-bottom flask.
- Add a fractionating column to the top of the round-bottom flask, securing it to the retort stand with a clamp.
- Add a "Y" adaptor to the top of the fractionating column, securing it to the retort stand with a clamp and to the fractionating column with a Keck clip.
- Add a condensing column to the downward sloping arm of the "Y" adaptor, securing it with a Keck clip. For additional stability, the condenser can be secured with a clamp to a second retort stand.
- Connect tubing from the water source to the lower connection on the condenser and attach the water return tube to the higher connection on the condenser. Ensure the tubing is securely fitted to the condenser (consider using copper wire to hold the tubing in place).
- Connect a collection adaptor to the open end of the condenser and secure the connection with a Keck clip.
- Have three 25 mL graduated cylinders available for collection of the distillate.
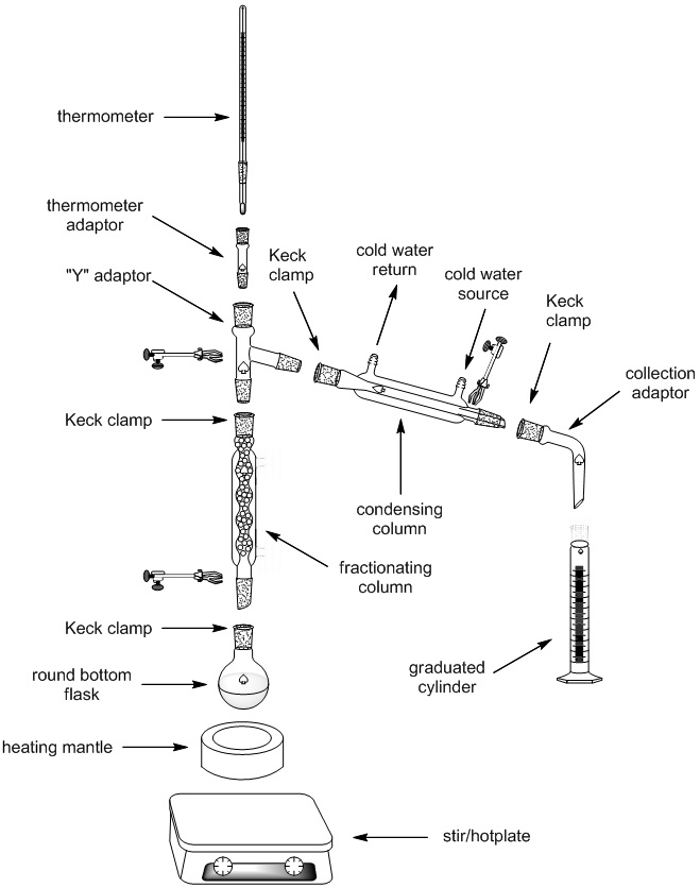
Figure 5. Apparatus for fractional distillation.
2. Preparation for the Distillation
- Remove the round bottom flask from the assembly by lowering it to the base of the retort stand.
- Place a stemmed funnel into the top of the round-bottom flask, and add the liquid to be distilled. Do not fill the flask more than half full.
- After filling the flask, remove the funnel.
- Raise the flask once again and place the flask back in its original position on the retort stand.
- Using the thermometer adaptor, place the thermometer into the remaining open port on the "Y" adaptor. The thermometer should be added last, as it is most susceptible to breakage.
- Position the bulb of the thermometer so that it is just below the side arm of the "Y" adaptor. This ensures an accurate reading of the vapor temperature.
- Replace the heating mantle and screw jack to their original position, snuggly enveloping the round-bottom flask.
- Place a graduated cylinder underneath the condenser adaptor, ready to collect the distillate.
3. Performing the Distillation
- Increase the temperature of the heating mantle gradually until the liquid in the round-bottom flask begins boiling.
- As soon as boiling commences, hold the temperature for 2 min.
- Note the ring of condensate rising slowly up the fractionating column. The rise should be gradual, which ensures proper separation of the mixture components across the theoretical plates. If the ring of condensate stops rising, increase the temperature slightly. It should take at least 5 min for the ring to reach the top of the column.
- Once the first drops of liquid start to fall down the condenser, the temperature should remain nearly constant. Record the temperature as each 2 mL of distillate collects in the graduated cylinder.
- Adjust the heat accordingly to allow for 1—2 drops/s from the condenser into the graduated cylinder.
- Each time the graduated cylinder reaches 4 mL, quickly replace it with an empty one.
- Pour the distillate into a clean, labeled vial for safekeeping.
- If the distillation is occurring very slowly, wrap the column with aluminum foil or glass wool to protect it from cold drafts.
- When the temperature begins to drop significantly, nearly all the cyclohexane has been distilled. Increase the temperature until the toluene starts to boil and distill. Again, record the temperature as each 2 mL of distillate collects.
- Before the entire content of the flask evaporates, stop heating the flask. It is important to ensure that the flask does not become dry, which might cause it to crack.
- Remove the heating mantle by lowering the screw jack to the base of the retort stand
- Analyze the contents of the vials of distillate to confirm the purity using a chosen technique, such as 1H NMR spectroscopy.
Distillation is one of the most commonly used techniques for purifying liquids in laboratory settings and accounts for up to 95% of all industrial separation processes.
This method separates and purifies liquids based on their volatility, or the tendency for its molecules to escape from the liquid phase as gaseous vapor. To distill a solution, it is heated until the most volatile compounds begin to vaporize. The resultant vapors are then condensed into a purified liquid, known as the distillate, and collected.
The most common distillation methods are: simple distillation, which employs just one vaporization-condensation cycle, and fractional distillation, which employs multiple vaporization-condensation cycles.
This video will review the principles behind simple and fractional distillation, typical laboratory distillation apparatuses, and demonstrate an example fractional distillation procedure. Finally, applications of distillation will be covered.
When a liquid is heated, the kinetic energy of its molecules increases, causing some of the molecules to transition from the liquid into the gaseous state. This process, called vaporization, increases the vapor pressure above the liquid relative to the atmospheric pressure. The temperature at which the liquid's vapor pressure equals the surrounding environment's air pressure, at which point 'bubbles' of vapor form within the liquid, is known as its boiling point. Above this temperature, the liquid will completely vaporize into a gas.
In a simple distillation, a mixture is heated in a flask, and the resultant vapors pass into a condensing column where they are cooled and condensed into a liquid called the distillate. This method uses just one vaporization-condensation cycle, and generates pure distillates from both mixtures of liquids and solids, and mixtures of liquids with vastly different boiling points.
Mixtures of liquids with boiling points differing by less than about 30 °C cannot be completely separated using simple distillation. The distillate composition, however, can be predicted using a 'boiling point-diagram', which plots temperature as a function of liquid and vapor composition. For example, an equal mixture of cyclohexane and toluene has a boiling point of 90 °C, resulting in a vapor composition of 80% cyclohexane and 20% toluene, and yielding an 80% pure distillate.
The boiling point diagram predicts that a second vaporization-condensation cycle, achieved by boiling the 80:20 distillate at roughly 84 °C, results in a 95% purity. Each consecutive cycle, referred to as a 'theoretical plate,' increases the purity of the, where 3 theoretical plates result in a 99% purity. Although it is possible to generate this by chaining multiple 'simple distillation' apparatuses together, 'fractional distillation' achieves this more efficiently.
The setup adds a "fractionating column," between the starting flask and the condenser. This column is typically filled with glass beads or metal wool, providing a large surface area for the liquid to condense and re-evaporate onto numerous times. This generates a large number of theoretical plates.
As vapors rise through the column, a ring of condensate forms and slowly moves up as the vapors separate across the theoretical plates. When the vapor reaches the top of the column, the molecules with the highest volatility pass into the condenser where they are collected as a high purity distillate.
Now that we've reviewed the basic principles behind distillation, let's walk through an example for a cyclohexane: toluene mixture.
Begin by assembling the components of the fractional distillation apparatus in the fume hood. Place the heating mantle and stir plate on top of the laboratory jack at the foot of the retort stand and raise everything 8 in. with the jack.
Attach three clamps to the retort stand. Position the round-bottom flask so that it fits snuggly into the heating mantle and secure it to the retort stand.
Add a magnetic stir bar to the flask, place the fractionating column on top, and secure it.
Fit the "Y" adaptor into the top of the fractionating column. Secure it to the retort stand with a clamp and then secure the ground-glass joint between it and the fractionating column with a keck clamp.
Fit the condenser into the downward sloping arm of the "Y" adaptor and secure the joint. For added stability, clamp the condensing column to a second retort stand.
Connect a tube from the water source to the bottom connection on the condenser. Connect a second tube from the upper condenser connection to the water return port.
Secure the tubing, turn on the water, and verify that water flows through the condenser.
Add a collection adaptor to the condensing column. Secure this joint with a keck clip and place a graduated cylinder beneath it.
Before installing the thermometer, lower the flask. Use a funnel to fill less than half of the flask with the starting mixture.
Raise the flask, fitting it back into the fractionating column, and securing it. Secure this final ground-glass joint with a keck clamp and then replace and reposition the jack and heating mantle.
Finally, fit the thermometer into an adaptor and place it into the remaining port of the "Y" adaptor. Adjust the height of the thermometer bulb, situating it just below the side arm of the "Y" adaptor to ensure accurate vapor temperature readings.
Turn on the hot plate, gradually increasing the temperature and heating the mantle until the starting mixture begins to boil. Adjust the hotplate temperature as needed to ensure that the mixture continues to boil, maintaining a constant vapor temperature for the first 2 min of the distillation.
Watch as the condensation ring forms, and rises, to the top of the column. When the first drops of liquid condense and drip into the graduated cylinder, record the vapor temperature. Keep the vapor temperature constant by adjusting the hotplate setting until drops fall from the condenser at a rate of 1–2 per second.
For each 2 mL increment of distillate volume, record the vapor temperature. After 4 mL has been collected, record the vapor temperature and then quickly replace the partially filled graduated cylinder with an empty one. Save the 4 mL sample in an individually labeled vial for future analysis.
Continue recording vapor temperatures at 2 mL intervals, and saving distillate samples at 4 mL intervals, until the vapor temperature drops significantly and the mixture stops boiling.
Turn off the hotplate and the water running to the condenser. The distillation of cyclohexane is now complete and the 4 mL distillate samples can be prepared for NMR analysis.
Evaluate the purity of both the starting mixture and the distillate using NMR spectroscopy, a common technique for assessing the composition and purity of mixtures. In our example, the NMR spectrum of the initial mixture shows the characteristic peaks associated with both toluene and cyclohexane. The NMR spectra of the first distillate we collected after fractional distillation, however, was pure cyclohexane.
Distillation has applications in a broad range of fields, from large-scale petroleum refineries to small-scale whiskey stills.
To generate distilled spirits such as vodka or whiskey, a mixture of grain fermentation products known as 'wash,' which is 10–12 % alcohol by volume, is boiled in a "still" and the resultant vapors separated by either simple or fractional distillation, depending on the still's design and the type of spirit. This allows 'the heart', ethanol, to be separated from 'the tails', like propanol and water, which have higher boiling points. Additionally, distillation allows for the elimination of 'the heads' like methanol, which famously caused blindness in poorly made moonshine. The distillate may be around 50 % alcohol if produced from simple distillation, or as much as 95 % if fractionally distilled.
Gas chromatography uses thousands, if not millions, of theoretical plates to separate volatile mixtures using fractional distillation on a micro-scale. Here, a mixture of volatiles used to stimulate the olfactory nerves of bees were injected into the gas chromatograph, which was used to separate and identify the compounds based on the amount of time they took to pass through the chromatography column.
Trace explosive vapors of TNT and RDX were selectively separated from a sample headspace using the principles of distillation. These samples were collected in temperature desorption tubes and introduced into a temperature desorption stage, where they were heated them to between 350 and 900 degrees to increase their volatility. Finally, they were selectively condensed using a cryotrap and introduced into a gas chromatograph for analysis.
You've just watched JoVE's introduction to fractional distillation. You should now understand the basic principles behind distillation, the apparatuses for simple and fractional distillation, and a basic fractional distillation procedure.
Thanks for watching!
Subscription Required. Please recommend JoVE to your librarian.
Results
Fractional Distillation of a Cyclohexane-Toluene Mixture
The purity of the distillate can be assessed by a number of techniques. One of the best is NMR spectroscopy. The 1H-NMR spectrum of the initial mixture prior to distillation is shown in Figure 6. Signals for both toluene and cyclohexane are clearly visible. 1HNMR spectra of the pure cyclohexane and pure toluene distillates are shown in Figures 7 and 8, respectively. No contaminants from the other component are seen in each case, showing the effectiveness of the distillation.

Figure 6.1H-NMR of a mixture of cyclohexane and toluene prior to distillation.

Figure 7.1H-NMR of the pure cyclohexane distillate.

Figure 8.1H-NMR of the pure toluene distillate.
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
Distillation accounts for about 95 % of all current industrial separation processes. The main difference between distillations performed on the laboratory-scale and those performed industrially is that the former are usually run in a batch-wise manner, whereas the latter are often run continuously. In continuous distillation, the starting mixture, vapors, and distillate are kept at a constant composition by carefully replenishing the staring material and removing fractions from both vapor and liquid in the system. The most widely used industrial application of continuous, fractional distillation is in petroleum refineries and natural gas processing facilities. At temperatures below 36 °C, natural gas separates from petroleum. Other substances, including petroleum ether and naphtha, separate before the petroleum reaches the 69–74 °C range, at which point gasoline separates.
Distillation also finds application in the food industry. It is used to produce a wide variety of alcoholic beverages, e.g., whiskey, rum, and brandy. When fruit and plant materials ferment, a dilute solution of ethanol is produced. Distilling the fermented material purifies and concentrates the ethanol. A variety of other components, such as fragrant esters and other types of alcohol, are also collected during the distillation process, which accounts for the unique flavor of the finished spirit.
Subscription Required. Please recommend JoVE to your librarian.




