2.3:
Elements: Chemical Symbols and Isotopes
An element is a pure substance that contains only one type of atom. Currently, 118 elements have been discovered, either occurring in nature or synthesized in a laboratory.
Elements vary widely in their abundance. The most abundant elements in the Earth’s crust are oxygen, silicon, aluminum, iron, and calcium. In comparison, the Earth’s atmosphere is mostly made up of nitrogen and oxygen.
On the periodic table, each square contains, at a minimum, an atomic number and a chemical symbol.
A chemical symbol is a one- or two-letter abbreviation given to an element. The first letter is capitalized and the second, if necessary, is lowercase to avoid confusion with compounds that contain two elements. Often, this symbol is the first letter or letters of the element’s English name. However, some symbols stem from Greek, Latin or German roots.
Additional details can be added to a chemical symbol. The atomic number, which is also the number of protons, is noted in the left subscript position. This number always is the same for a given element and is sometimes omitted, as it does not change.
The mass number — the number of protons plus neutrons — is indicated in the left superscript position. This number defines the specific isotope of an element, such as carbon-12 or carbon-13.
An isotope is a variation of an element where the number of neutrons in the nucleus varies but the number of protons — also the atomic number — is the same. This results in different atomic masses but does not affect the charge of the atom. For instance, elemental hydrogen has three naturally occurring isotopes: The most abundant form contains no neutrons; the second is deuterium, containing one neutron; and the third is tritium, which contains two neutrons.
While the standard hydrogen atom and deuterium are stable under natural conditions, other isotopes, such as tritium, decay into other substances by the emission of energy. These are known as radioactive isotopes.
2.3:
Elements: Chemical Symbols and Isotopes
A chemical symbol is an abbreviation used to indicate an element or an atom of an element. For example, the symbol for mercury is Hg. The same symbol is used to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).
Some symbols are derived from the common English name of the element; others are abbreviations of the name in another language — Latin, Greek or German. For example, the symbol for aluminum (common name) is Al, while that for Iron is Fe which stems from its Latin name “Ferrum”. Most symbols have one or two letters, but three-letter symbols have been used to describe some elements that have atomic numbers greater than 112. To avoid confusion with other notations, only the first letter of a symbol is capitalized. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O).
Traditionally, the discoverer (or discoverers) of a new element names the element. However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number. For example, element 106 was called unnilhexium (Unh), element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named after scientists (or occasionally locations); for example, element 106 is now known as seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.
Each square on the periodic table contains, at minimum, an atomic number — which is also the number of protons — and a chemical symbol.
In the case of an isotope, the mass number or the sum of protons and neutrons is indicated along with the symbol and atomic number. The mass number defines the specific isotope of an element. For example, these are the two isotopes of nitrogen:
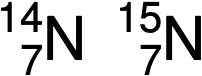
The symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol. Since the atomic number defines the element’s identity, as does its symbol, it is often omitted. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24Mg, 25Mg, and 26Mg. These isotope symbols are read as “element, mass number” and can be symbolized consistent with this reading. For instance, 24Mg is read as “magnesium 24” and can be written as “magnesium-24” or “Mg-24.” 25Mg is read as “magnesium 25,” and can be written as “magnesium-25” or “Mg-25.” All magnesium atoms have 12 protons in their nucleus. They differ only because a 24Mg atom has 12 neutrons in its nucleus, a 25Mg atom has 13 neutrons, and a 26Mg has 14 neutrons.
Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3H, is also called tritium and sometimes symbolized T.
Text adapted from Openstax Chemistry 2e, Section 2.3: Atomic Structure and Symbolism.
Suggested Reading
- Pothoof, Justin, Grace Nguyen, Dawn Archey, E. Prasad Venugopal, and Mark A. Benvenuto. "Element 118: Teaching A New Element to New Students." In Elements Old and New: Discoveries, Developments, Challenges, and Environmental Implications, pp. 195-201. American Chemical Society, 2017.
- Krebs, Robert E. The history and use of our earth's chemical elements: a reference guide. Greenwood Publishing Group, 2006.
- Clayton, Donald. Handbook of isotopes in the cosmos: Hydrogen to gallium. Vol. 1. Cambridge University Press, 2003.
