4.1:
Reaction Stoichiometry
Consider a balanced chemical reaction, such as the combustion of hydrogen gas. Here, the quantitative relationship between the reactants and products — H2, O2, and H2O — is that two molecules of H2 react with one molecule of O2 to produce two molecules of H2O.
This quantitative relationship is known as stoichiometry, and it is similar to any recipe.
Suppose two slices of salami, one slice of cheese, and two pieces of Italian bread are used to make one sandwich. In order to make three sandwiches, the quantities of the ingredients are tripled.
How many sandwiches could be made with 10 slices of salami? Since the ratio of salami to sandwiches is two to one, five sandwiches could be made.
This same process is applied to chemical reactions. For example, consider the synthesis of ammonia. The stoichiometric coefficients directly indicate the relative number of molecules, which is equivalent to the relative quantities in moles.
One mole of nitrogen gas and three moles of hydrogen gas react to form two moles of ammonia. To make four moles of ammonia, the reactant quantities are doubled.
The mole ratio of nitrogen gas to ammonia is one to two, while that of hydrogen gas to ammonia is three to two. If there are 15 moles of hydrogen, how many moles of ammonia can be synthesized? Using the mole ratio as a conversion factor, 10 moles of ammonia can be synthesized.
To estimate the mass of a reactant from the mass of the product or vice versa, a pathway is followed. Unlike mole conversions, calculations involving mass are not direct. First, the known mass is converted to moles. Then, the mole ratio is applied. Lastly, the amount in moles is converted to mass using the molar mass of the relevant compound.
For example, consider the combustion of a hydrocarbon rocket propellant – that is, its reaction with oxygen. Approximately how many grams of liquid oxygen are required for each 5,000 grams of fuel?
First, the approximate molar mass of the fuel is used to convert 5,000 grams to moles. Then, the mole ratio of 35 to 2 is applied to calculate the needed amount in moles of molecular oxygen. Finally, the molar mass of molecular oxygen is used to determine that the spacecraft needs to store about 17,000 grams of oxygen onboard for each 5,000 grams of fuel.
4.1:
Reaction Stoichiometry
A balanced chemical equation provides a great deal of information in a very succinct format. Chemical formulas provide the identities of the reactants and products involved in the chemical change, allowing classification of the reaction. Coefficients provide the relative numbers of these chemical species, allowing a quantitative assessment of the relationships between the amounts of substances consumed and produced by the reaction. These quantitative relationships are known as the reaction’s stoichiometry, a term derived from the Greek words stoicheion (meaning “element”) and metron (meaning “measure”).
A reaction’s stoichiometry helps predict how much of the reactant is needed to produce the desired amount of product, or in some cases, how much product will be formed from a specific amount of the reactant.
Stoichiometric Coefficients
The coefficients of a chemical equation represent the number of moles of each substance. For example, consider the reaction of nitrogen gas and hydrogen gas to produce ammonia. Stoichiometry indicates that one mole of nitrogen and three moles of hydrogen react to produce two moles of ammonia.

Since one mole contains Avogadro's number of molecules, the relative numbers of molecules are the same as the relative numbers of moles. An alternative way of reading the equation is “one molecule of nitrogen and three molecules of hydrogen react to produce two molecules of ammonia.”
Stoichiometric Factors
Balanced chemical equations are used to determine the amount of one reactant required to react with a given amount of another reactant, or to yield a given amount of product, and so forth. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. In the reaction of hydrogen and nitrogen, ammonia molecules are produced from hydrogen molecules in a 2:3 ratio. This means three moles of hydrogen are stoichiometrically equivalent to two moles of ammonia.
Based on this, the following stoichiometric factors are derived:
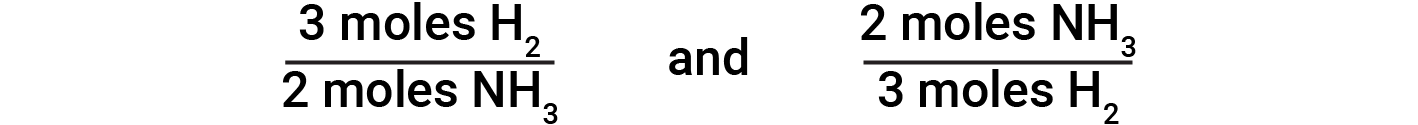
These stoichiometric factors can be used to compute the number of ammonia molecules produced from a given number of hydrogen molecules, or the number of hydrogen molecules required to produce a given number of ammonia molecules. Similar factors may be derived for any pair of substances in any chemical equation.
Mole-to-Mole Conversions
For a balanced chemical reaction for the formation of ammonia from nitrogen and hydrogen, the following stoichiometric mole ratio between N2 and NH3 is 1:2. Then, the molar amount of ammonia is derived by multiplying the molar amount of nitrogen by the stoichiometric conversion factor relating the two substances of interest.

Mass-to-Mass Conversions
Converting between masses of substances based on stoichiometry requires the knowledge of mole ratios and molar masses. For example, to find the mass of hydrogen required to produce 0.170 kg of ammonia, first, the molar mass of ammonia is used to convert the mass of ammonia to the amount of ammonia (in moles). Then, the appropriate stoichiometric factor from the balanced equation converts the amount of ammonia (in moles) to the amount of hydrogen (in moles). Lastly, the molar mass of hydrogen converts the amount of hydrogen (in moles) to the mass of hydrogen.
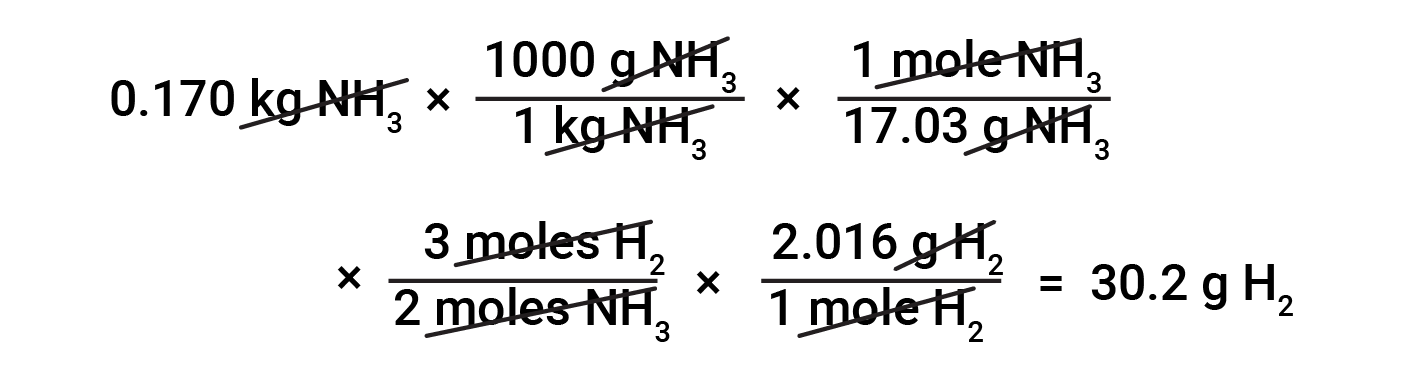
Numerous variations on the beginning and ending computational steps are possible depending upon what particular quantities are provided and sought (volumes, solution concentrations, and so forth). Regardless of the details, all these calculations share a common essential component: the use of stoichiometric factors derived from balanced chemical equations.
This text is adapted from OpenStax Chemistry 2e, Section 4.3: Reaction Stoichiometry.
