4.10:
Oxidation-Reduction Reactions
Certain processes that are vital to life, including photosynthesis, combustion, and corrosion, fall into the class of reactions called oxidation–reduction, or redox, reactions.
Redox reactions consist of two simultaneous processes: oxidation and reduction.
The term oxidation means an increase in oxidation number, which corresponds to the loss of electrons, while reduction means a decrease in oxidation number, which corresponds to the gain of electrons. To remember the role of electrons, use the acronym OIL RIG, which stands for: “oxidation is losing, reduction is gaining.”
Oxidation and reduction are complementary processes. In a redox reaction between two reactants, one reactant loses electrons and is oxidized, while the other reactant gains electrons and is reduced.
Consider the oxidation–reduction reaction between potassium — an alkali metal — and chlorine, a nonmetal.
The neutral potassium atom loses an electron to become a potassium ion. Potassium is oxidized, and its charge increases from zero in the neutral atom to one-plus in the cation.
The neutral chlorine atom gains an electron and becomes a chloride ion. Chlorine is reduced, and its charge decreases from zero in the neutral atom to one-minus in the anion.
Since potassium donates an electron, it is the reducing agent, or reductant. Chlorine accepts the electron, so it is the oxidizing agent, or an oxidant. The redox process leads to the formation of potassium chloride.
In general, in redox reactions between alkali or alkaline earth metals and nonmetals, the metal is oxidized and the nonmetal is reduced to form an ionic compound through complete electron transfer. This is often true for reactions between other metals or metalloids and nonmetals as well, but not always.
Another example of a redox process is the formation of gaseous hydrogen chloride. Here, both reactants — hydrogen and chlorine — are nonmetals, so there is no complete transfer of electrons. Instead, hydrogen shares an electron with chlorine in a partial, or formal, electron transfer.
Thus, in the formation of hydrogen chloride, hydrogen is oxidized and acquires a partial positive charge, while chlorine is reduced and acquires a partial negative charge. Since both oxidation and reduction processes occur, this is a redox reaction.
In general, redox reactions between nonmetals involve partial electron transfer between elements to form a covalent compound.
4.10:
Oxidation-Reduction Reactions
Oxidation–Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation–reduction (redox) reactions.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:

It is helpful to view the process with regard to each individual reactant, that is, to represent the fate of each reactant in the form of an equation called a half-reaction:

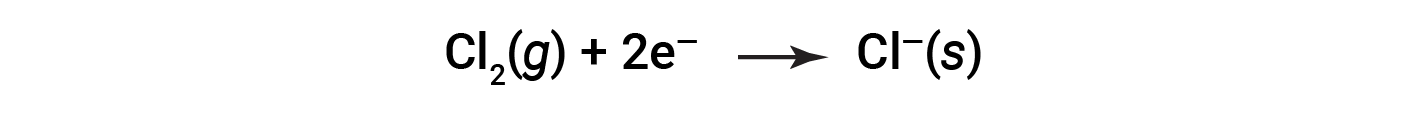
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “s” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
oxidation = loss of electrons
reduction = gain of electrons
In this reaction, sodium is oxidized and chlorine undergoes reduction. Viewed from a more active perspective, sodium functions as a reducing agent (reductant), since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant), as it effectively removes electrons from (oxidizes) sodium.
reducing agent = species that is oxidized
oxidizing agent = species that is reduced
In general, an oxidizing agent gains an electron from the reducing agent, and itself gets reduced. The charge of an oxidizing agent becomes more negative. Similarly, a reducing agent loses an electron to the oxidizing agent, and itself gets oxidized. The charge of a reducing agent becomes more positive.
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
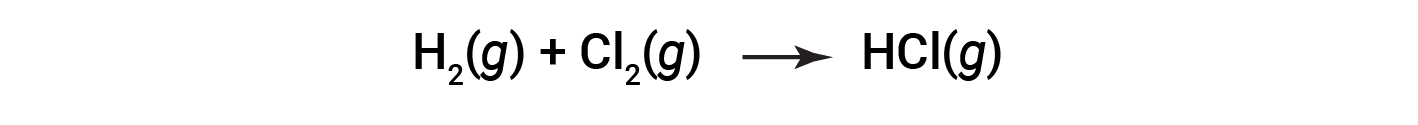
The product of this reaction is a covalent compound, so the transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined.
This text is adapted from Openstax, Chemistry 2e, Section 4.2: Classifying Chemical Reactions.
