4.9:
Precipitation Reactions
Ionic solids may be water-soluble or water-insoluble. Water-soluble ionic solids dissolve by breaking up — or dissociating — into ions. Water molecules then surround the dissociated ions to form an aqueous solution. Water-insoluble ionic solids stay undissolved or undissociated in aqueous solutions.
If mixing aqueous solutions of two different ionic compounds results in a chemical reaction that produces an insoluble ionic solid — called the precipitate — it is a precipitation reaction. The precipitate stays undissolved and can be separated from the solution by filtration.
Sodium chloride and silver nitrate are water-soluble ionic compounds. An aqueous solution of sodium chloride contains sodium ions and chloride ions in their dissociated forms. Similarly, an aqueous solution of silver nitrate contains dissociated silver ions and nitrate ions.
When the two solutions are mixed, the resulting solution contains all four ions: the sodium and silver cations as well as the chloride and nitrate anions.
Since oppositely charged ions are attracted to each other, the cation from each reactant can pair with the anion from the other reactant to form new ionic products. Thus, sodium cations pair with nitrate anions to form sodium nitrate, and silver cations combine with chloride anions to form silver chloride.
Referring to the solubility rules allows us to determine whether the ionic products are water-soluble or water-insoluble. According to this guide, all nitrate salts are water-soluble without exception. Most chloride salts are water-soluble, but silver salt is an exception.
Therefore, sodium nitrate is a soluble salt that remains in the solution as dissolved sodium ions and nitrate ions, while silver chloride is an insoluble salt that precipitates from the solution.
The molecular equation for this precipitation reaction suggests that aqueous sodium chloride reacts with aqueous silver nitrate to form aqueous sodium nitrate and solid silver chloride.
This is an example of a salt metathesis reaction, which is also called a double displacement reaction. Salt metathesis is a chemical process in which the positive and negative ions of two ionic reactants exchange partners to form two new ionic products.
In this reaction, sodium and silver cations exchange partners — pairing with nitrate and chloride anions, respectively — to form sodium nitrate and silver chloride.
4.9:
Precipitation Reactions
In a precipitation reaction, aqueous solutions of soluble salts react to give an insoluble ionic compound – the precipitate. The reaction occurs when oppositely charged ions in solution overcome their attraction for water and bind to each other, forming a precipitate that separates out from the solution. Since such reactions involve the exchange of ions between ionic compounds in aqueous solution, they are also referred to as double displacement, double replacement, exchange reactions, or metathesis reactions (Greek for “to transpose”). A precipitation reaction is used as an analysis technique to identify metal ions in a compound and gravimetric methods for determining the composition of matter.
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble, and these are the substances that readily precipitate from solution.
For example, precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead iodide:

This observation is consistent with the solubility guidelines: The only insoluble compound among all those involved is lead iodide, one of the exceptions to the general solubility of iodide salts.
The net ionic equation representing this reaction is:
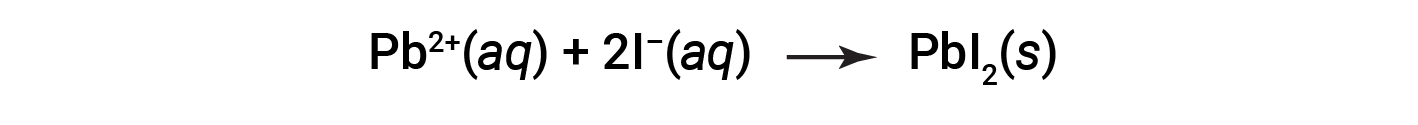
The solubility guidelines may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. One merely needs to identify all the ions present in the solution and then consider if possible cation-anion pairing could result in an insoluble compound.
For example, mixing solutions of silver nitrate and sodium fluoride will yield a solution containing Ag+, NO3−, Na+, and F− ions. Aside from the two ionic compounds originally present in the solutions, AgNO3 and NaF, two additional ionic compounds may be derived from this collection of ions: NaNO3 and AgF. The solubility guidelines indicate all nitrate salts are soluble but that AgF is one of the exceptions to the general solubility of fluoride salts. A precipitation reaction, therefore, is predicted to occur, as described by the following equations:


This text is adapted from OpenStax Chemistry 2e, Section 4.2: Classifying Chemical Reactions.
