6.7:
Thermochemical Equations
Most chemical reactions occur at atmospheric pressure. Under conditions of constant pressure, the heat change associated with the reaction, ΔQ, equals the change in enthalpy, ΔH, also called the enthalpy or heat of reaction.
Enthalpy of the reaction is the difference between the enthalpies of the products and the reactants. When the enthalpy of the products is greater than the enthalpy of the reactants, ΔH is positive. Such reactions absorb heat and are endothermic.
On the contrary, if the enthalpy of the reactants is greater than the enthalpy of the products, ΔH is negative. Such reactions release heat and are exothermic.
For any chemical reaction, the magnitude of the accompanying enthalpy change depends on the stoichiometric amounts of reactants and products, as indicated by the coefficients of the balanced equation.
A balanced chemical equation that includes phase labels and enthalpy of reaction, ΔH, is called a thermochemical equation.
Consider the combustion of methane – a primary source of fuel. Burning methane releases heat to the surroundings. The exothermic nature of the reaction is indicated by the negative enthalpy change in the thermochemical equation.
The equation for combustion shows that when one mole of methane gas reacts with 2 moles of oxygen gas to yield 1 mole of carbon dioxide gas and 2 moles of liquid water, 890.8 kilojoules of heat are released to the surroundings.
The molar ratio between the reactants or products and the heat of reaction can be used as conversion factors to calculate the heat exchanged during the reaction.
If a gas cylinder contains 25.5 kilograms of methane, and all of the methane in the cylinder undergoes combustion, what amount of heat will be produced? In general, the conceptual plan is to convert the mass to moles and then moles to the heat of reaction.
To begin, 25.5 kilograms is multiplied by 1000 to find the mass in grams. Then, the mass of methane is divided by its molar mass – 16.0 grams per mol, to yield 1594 moles of methane.
Finally, using the conversion factor between moles of methane and heat of reaction, 1594 moles of methane releases 14.2 times ten to the 6 kilojoules of heat – which is the heat of the reaction. The answer is negative since the reaction is exothermic as heat evolves in the reaction.
6.7:
Thermochemical Equations
For a chemical reaction (the system) carried out at constant pressure – with the only work done caused by expansion or contraction – the enthalpy of reaction (also called the heat of reaction, ΔHrxn) is equal to the heat exchanged with the surroundings (qp).
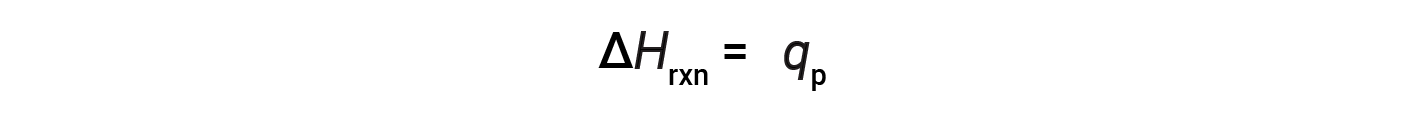
The change in enthalpy is an extensive property, and it depends on the amounts of the reactants participating in the reaction (or the number of moles of reactants). The change in enthalpy is specific to the reaction, and the physical states of the reactant and product species are important. An exothermic reaction is characterized by a −ΔHrxn value, while an endothermic reaction has a +ΔHrxn value.
Because the amount of heat released or absorbed by a reaction corresponds to the amount of each substance consumed or produced by the reaction, it is convenient to use a thermochemical equation to represent the changes in both matter and energy. In a thermochemical equation, the change in enthalpy of a reaction is shown as ΔHrxn, and it is generally provided following the equation for the reaction. The magnitude of ΔHrxn indicates the amount of heat associated with the reaction shown in the chemical equation. The sign of ΔHrxn indicates if the reaction is exothermic or endothermic, as written. In the following equation, 1 mole of hydrogen gas and 1/2 mole of oxygen gas (at some temperature and pressure) react to form 1 mole of liquid water (at the same temperature and pressure).
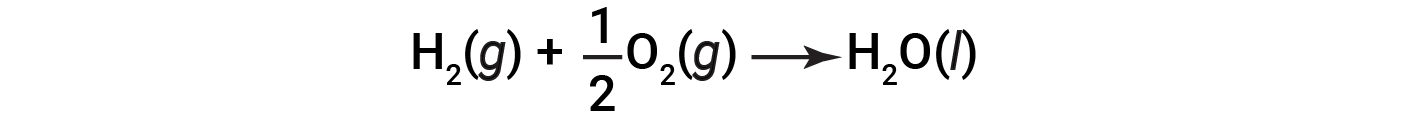
This equation indicates that 286 kJ of heat is released to the surroundings. In other words, 286 kJ of heat is released (reaction is exothermic) for every mole of hydrogen that is consumed or for every mole of water that is produced. Therefore, the enthalpy of reaction is a conversion factor that can be used to calculate the amount of heat that is released or absorbed during reactions involving specific amounts of reactants and products.
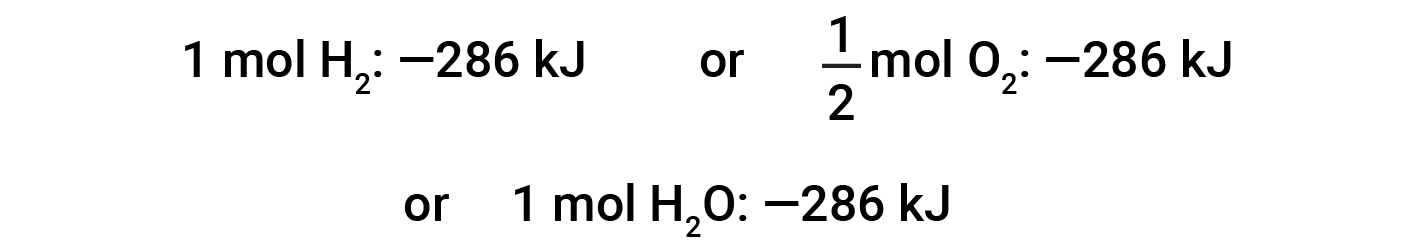
If the coefficients of the chemical equation are multiplied by some factor (i.e., if the amount of a substance is changed), the change in enthalpy must be multiplied by that same factor.
(two-fold increase in amounts)
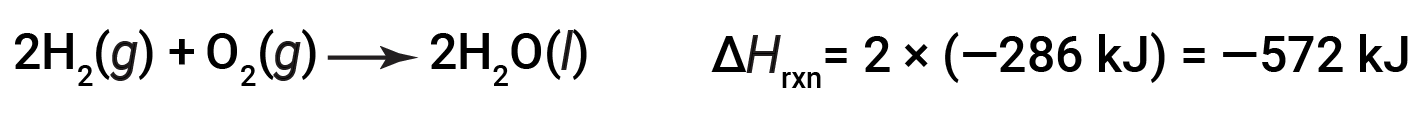
(two-fold decrease in amounts)
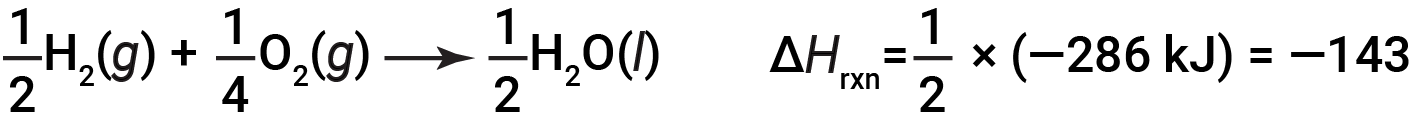
To illustrate that the enthalpy change of a reaction depends on the physical states of the reactants and products, consider the formation of gaseous water (or water vapor). When 1 mole of hydrogen gas and ½ mole of oxygen gas react to form 1 mole of gaseous water, only 242 kJ of heat are released, as opposed to 286 kJ of heat, which is released when liquid water forms.
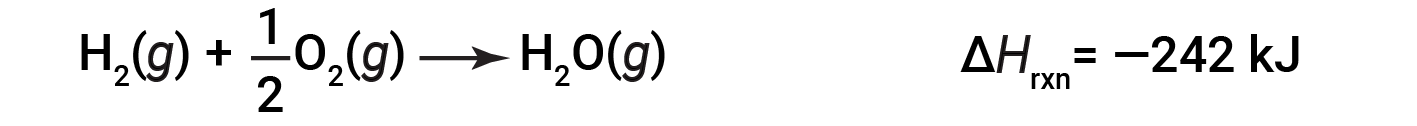
Suggested Reading
- Canagaratna, Sebastian G. "A visual aid in enthalpy calculations." Journal of Chemical Education 77, no. 9 (2000): 1178.
- Keifer, David. "Enthalpy and the Second Law of Thermodynamics." Journal of Chemical Education 96, no. 7 (2019): 1407-1411.
- Khalil, Mutasim I. "Calculating enthalpy of reaction by a matrix method." Journal of Chemical Education 77, no. 2 (2000): 185.
