6.10:
Hessscher Wärmesatz
6.10:
Hessscher Wärmesatz
There are two ways to determine the amount of heat involved in a chemical change: measure it experimentally, or calculate it from other experimentally determined enthalpy changes. Some reactions are difficult, if not impossible, to investigate and make accurate measurements for experimentally. And even when a reaction is not hard to perform or measure, it is convenient to be able to determine the heat involved in a reaction without having to perform an experiment.
This type of calculation usually involves the use of Hess’s law, which states: If a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps. Hess’s law is valid because enthalpy is a state function: Enthalpy changes depend only on where a chemical process starts and ends, but not on the path it takes from start to finish. For example, the reaction of carbon with oxygen to form carbon dioxide occurs either directly or by a two-step process. The direct process is written as:
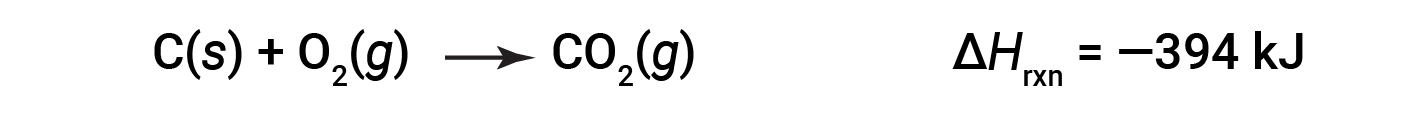
In the two-step process, first carbon monoxide is formed:
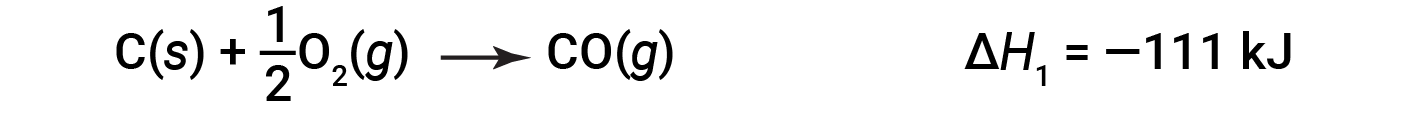
Then, carbon monoxide reacts further to form carbon dioxide:
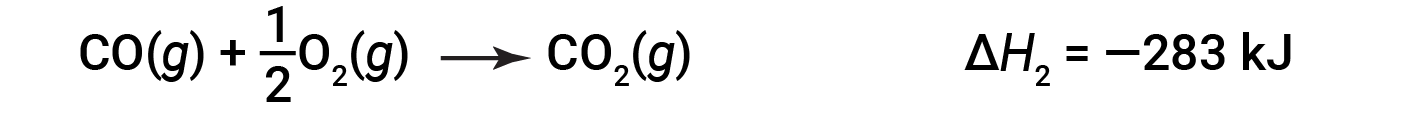
The equation describing the overall reaction is the sum of these two chemical changes:
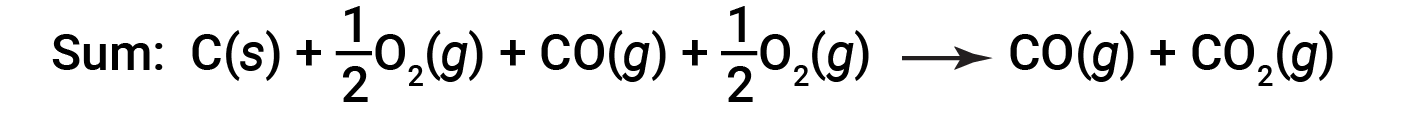
Because the CO produced in Step 1 is consumed in Step 2, the net change is:

According to Hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps.

ΔH of the overall reaction is the same, regardless of whether it occurs in one step or two. This finding (overall ΔH for the reaction = sum of ΔH values for reaction “steps” in the overall reaction) is true in general for chemical and physical processes.
There are two important features of ΔH that prove useful while solving problems using Hess’s law. This is based on the fact that ΔH is directly proportional to the quantities of reactants or products, and changing the reaction (or the thermochemical equation) in well-defined ways changes the ΔH accordingly.
For example, the enthalpy change for the reaction forming 1 mole of NO2 (g) is +33.2 kJ:
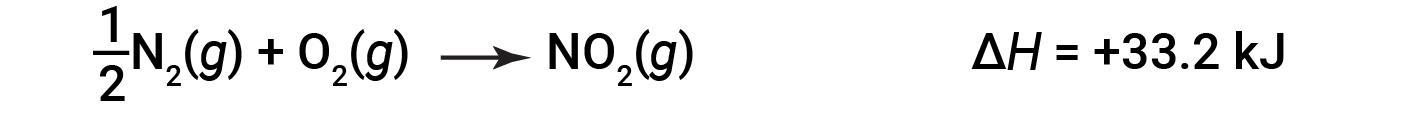
When 2 moles of NO2 (twice as much) are formed, the ΔH is twice as large:
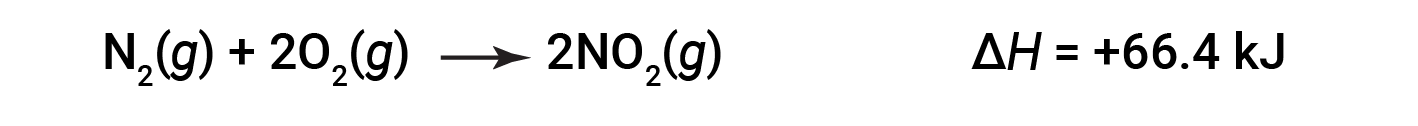
In general, if multiplying or dividing a chemical equation, the change in enthalpy should also be multiplied or divided by the same number.
ΔH for a reaction in one direction is equal in magnitude and opposite in sign to ΔH for the reaction in the reverse direction. For example:
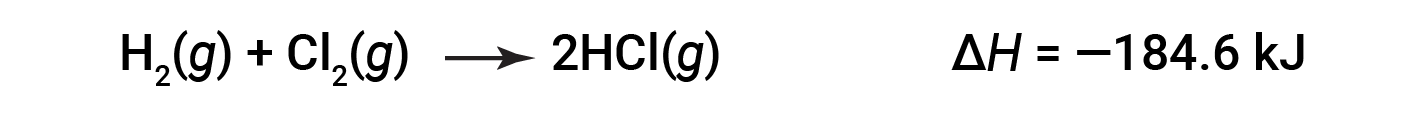
Then, for the reverse reaction, the enthalpy change is also reversed:
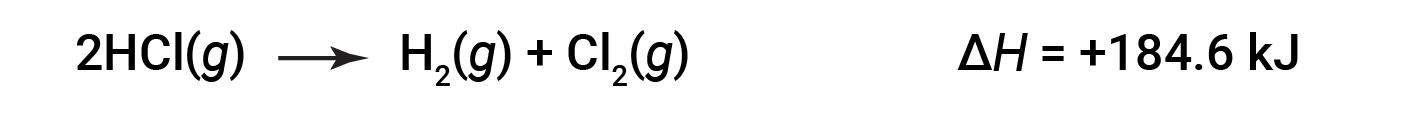
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Suggested Reading
- Davis, Thomas W. "A common misunderstanding of Hess' law." Journal of Chemical Education 28, no. 11 (1951): 584.
- Lee, A. L., H. L. Feldkirchner, F. C. Schora, and J. J. Henry. "Heat of Reaction of Hydrogen and Coal." Industrial & Engineering Chemistry Process Design and Development 7, no. 2 (1968): 244-249.
