6.11:
Standard Enthalpy of Formation
The enthalpy change of a reaction can be measured using a calorimeter, or it can be found by calculating the difference in enthalpy between the reactants and the products.
However, the absolute enthalpies of the reactants and products cannot be measured directly; therefore, chemists generally use the change in enthalpy, or ΔH, relative to a reference substance in an agreed-upon standard state.
The standard state is defined by a specific set of conditions. This includes a set temperature, usually 25 °C or 298 K, and a constant pressure of 1 bar for gases.
For solutions, the standard state is a 1 molar concentration of a pure solute in a solvent.
The standard state for a substance also includes the physical state of matter in which the substance exists under these conditions. For example, sodium chloride as a solid, mercury as a liquid, or helium as a gas.
If an element exists in more than one form under these conditions, the most stable form of the element is defined as the standard state. For example, carbon can exist as graphite crystals or as a diamond, but graphite is the most stable form and therefore, the standard state of carbon.
When elements in their standard states combine to form 1 mole of a pure compound, the enthalpy of the reaction is called standard enthalpy, or standard heat, of formation. This is denoted by ΔHf°, where naught indicates the standard states of the constituent elements, while f indicates formation.
The standard enthalpy of formation for pure elements under standard state conditions is always zero because there is no reaction, and therefore no change in enthalpy, when the element is already in its standard state.
The values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. These substances include elements in non-standard states, such as gaseous sodium, and compounds such as sodium chloride.
The standard enthalpy change of a reaction can be calculated from the difference of products and reactants, which in turn, can be calculated by using the enthalpy values from the reference table.
6.11:
Standard Enthalpy of Formation
Enthalpy changes are typically tabulated for reactions in which both the reactants and products are at the same conditions. A standard state is a commonly accepted set of conditions used as a reference point for the determination of properties under other different conditions. For chemists, the IUPAC standard state refers to materials under a pressure of 1 bar and solutions at 1 M and does not specify a temperature. Many thermochemical tables list values with a standard state of 1 atm. Because the ΔH of a reaction changes very little with such small changes in pressure (1 bar = 0.987 atm), ΔH values (except for the most precisely measured values) are essentially the same under both sets of standard conditions. A superscripted “o” in the enthalpy change symbol designates standard state. Since the usual (but not technically standard) temperature is 298.15 K, this temperature will be assumed unless some other temperature is specified. Thus, the symbol (ΔH°) is used to indicate an enthalpy change for a process occurring under these conditions. (The symbol ΔH is used to indicate an enthalpy change for a reaction occurring under nonstandard conditions.)
The enthalpy changes for many types of chemical and physical processes are available in the reference literature, including those for combustion reactions, phase transitions, and formation reactions. Since the enthalpy change for a given reaction is proportional to the amounts of substances involved, it may be reported on that basis (i.e., as the ΔH for specific amounts of reactants). However, we often find it more useful to divide one extensive property (ΔH) by another (amount of substance), and report a per-amount intensive value of ΔH, often “normalized” to a per-mole basis.
Standard Enthalpy of Formation
The standard enthalpy of formation ΔHf° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free elements in their most stable states under standard state conditions. These values are especially useful for computing or predicting enthalpy changes for chemical reactions that are impractical or dangerous to carry out, or for processes for which it is difficult to make measurements. Using known values of standard enthalpies of formation, the enthalpy change for any reaction can be determined.
The standard enthalpy of formation of CO2 (g) is −393.5 kJ/mol. This is the enthalpy change for the exothermic reaction:
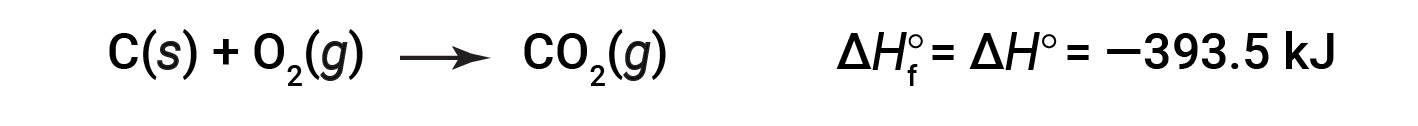
starting with the reactants at a pressure of 1 atm and 25 °C (with the carbon present as graphite, the most stable form of carbon under these conditions) and ending with one mole of CO2, also at 1 atm and 25 °C. For nitrogen dioxide, NO2 (g), ΔHf° is 33.2 kJ/mol. This is the enthalpy change for the endothermic reaction:
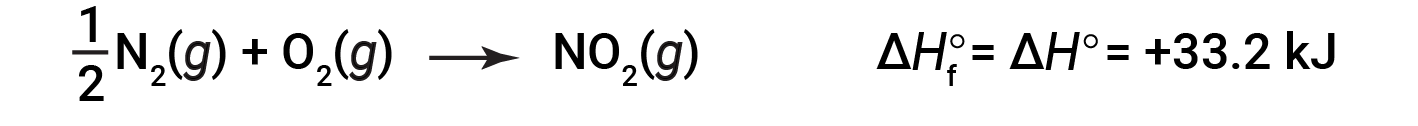
A reaction equation with 1/2 mole of N2 and 1 mole of O2 is correct in this case because the standard enthalpy of formation always refers to 1 mole of product: NO2 (g).
By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions. For example, the standard enthalpies of formation of carbon (graphene), diatomic oxygen gas, diatomic nitrogen gas, sodium metal, and liquid mercury are zero under standard conditions.
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Suggested Reading
- Hawk, Eric Leigh. "The calculation of standard enthalpies of formation of alkanes: Illustrating molecular mechanics and spreadsheet programs." Journal of chemical education 76, no. 2 (1999): 278.
- Mazzuca, James W., Alexis R. Downing, and Christopher Potter. "Empirically corrected electronic structure calculations applied to the enthalpy of combustion physical chemistry laboratory." Journal of Chemical Education 96, no. 6 (2019): 1165-1170.
- Jansen, Michael P. "The Cost of Converting a Gasoline-Powered Vehicle to Propane. A Practical Review Problem for Senior High School or Introductory Chemistry." Journal of Chemical Education 77, no. 12 (2000): 1578.
