6.12:
Enthalpies of Reaction
For a reaction occurring under standard conditions, a general equation is used to calculate the standard enthalpy change of the reaction. This equation is solved by finding the difference between the sum of the standard enthalpies of formation of the products and the sum of the standard enthalpies of formation of the reactants, each multiplied by its stoichiometric coefficient.
Consider the combustion of 2 moles of acetylene gas with 5 moles of oxygen gas to form 4 moles of carbon dioxide gas and 2 moles of water vapor, under standard conditions.
The enthalpy for the reaction equals the sum of four times the enthalpy of formation of carbon dioxide gas and two times the enthalpy of formation of water vapor, minus the sum of two times the enthalpy of formation of acetylene gas and five times the enthalpy of formation of oxygen gas.
The enthalpy equation is derived from combining two concepts: standard enthalpy of formation and Hess’s law.
The first term represents the standard enthalpies of formation of the products; the formation of carbon dioxide from carbon and oxygen — equation 1, and the formation of water from hydrogen and oxygen — equation 2.
The known standard enthalpies of formation for carbon dioxide and water are −393.5 kJ and −241.8 kJ, respectively.
Since the combustion produces 4 moles of carbon dioxide, ΔH1 would be the standard enthalpy of formation of carbon dioxide times 4, which would be −574 kJ.
The combustion also produces 2 moles of water, so the enthalpy change, ΔH2, would be the standard enthalpy of formation of water times 2, which would be −483.6 kJ.
This yields −2058 kJ as the net standard enthalpy of formation of the products.
The second term represents the decomposition of acetylene into carbon and hydrogen — equation 3. This is the reverse of the reaction for the standard enthalpy of formation of the reactant, and thus, its enthalpy value, 227.4 kJ, is preceded by a negative sign, which can also be seen in the enthalpy equation.
Because the reaction consumes 2 moles of acetylene, ΔH3 would be the negative of the standard enthalpy of formation of acetylene times 2, which equals − 53.4 kJ.
Standard formation enthalpy of oxygen is zero.
Therefore, the net standard enthalpy of formation of the reactants is −453.4 kJ.
Recall from Hess’s Law, that if a one-step reaction is carried out in multiple steps, then the sum of the enthalpies of each step, equals the net enthalpy change.
Substituting the values for the enthalpies of formation into the equation gives the enthalpy of the reaction as −2511 kJ.
6.12:
Enthalpies of Reaction
Hess’s law can be used to determine the enthalpy change of any reaction if the corresponding enthalpies of formation of the reactants and products are available. The main reaction may be divided into stepwise reactions : (i) decompositions of the reactants into their component elements, for which the enthalpy changes are proportional to the negative of the enthalpies of formation of the reactants, −ΔHf°(reactants), followed by (ii) re-combinations of the elements (obtained in step 1) to give the products, with the enthalpy changes proportional to the enthalpies of formation of the products, ΔHf° (products). The standard enthalpy change of the overall reaction is therefore equal to: (ii) the sum of the standard enthalpies of formation of all the products plus (i) the sum of the negatives of the standard enthalpies of formation of the reactants, as given by the following equation, where ∑ represents “the sum of” and n stands for the stoichiometric coefficients.
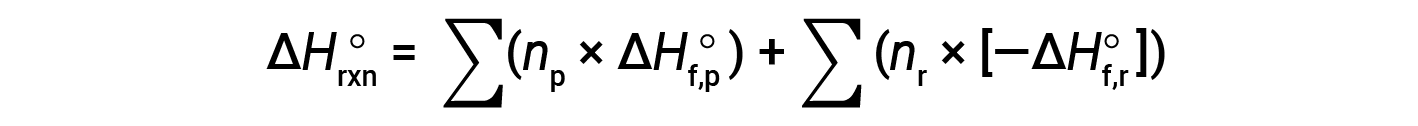
The equation is usually rearranged slightly to be written as follows:
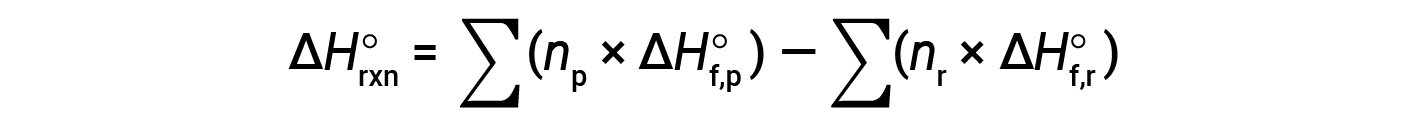
The following example shows in detail why this equation is valid and how to use it to calculate the standard enthalpy change for a reaction:

Here, the special form of Hess’s law and the heat of formation values for the reactants and products is used: ΔHf° (HNO3) = −206.64 kJ/mol; ΔHf° (NO) = +90.2 kJ/mol; ΔHf° (NO2) = +33 kJ/mol; ΔHf° (H2O) = −285.8 kJ/mol.


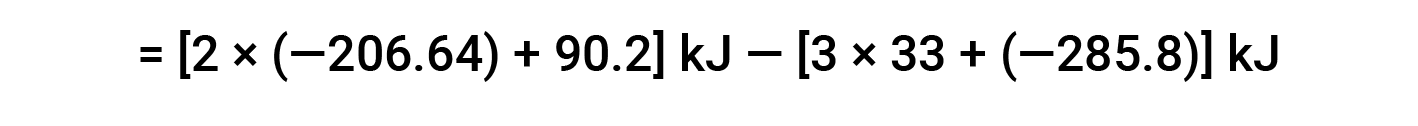
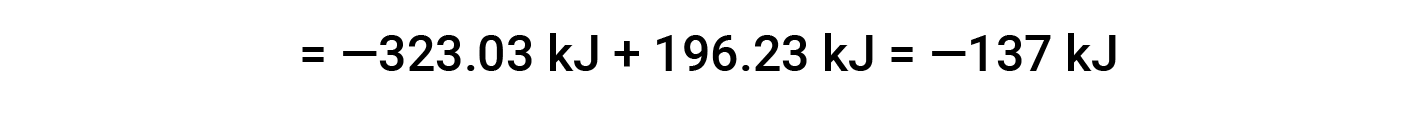
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Suggested Reading
- Davis, Thomas W. "A common misunderstanding of Hess' law." Journal of Chemical Education 28, no. 11 (1951): 584.
- Lee, A. L., H. L. Feldkirchner, F. C. Schora, and J. J. Henry. "Heat of Reaction of Hydrogen and Coal." Industrial & Engineering Chemistry Process Design and Development 7, no. 2 (1968): 244-249.
