10.9:
Molekülorbitaltheorie II
10.9:
Molekülorbitaltheorie II
Molecular Orbital Energy Diagrams
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram. For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Dashed lines show which of the atomic orbitals combine to form the molecular orbitals. For each pair of atomic orbitals that combine, one lower-energy (bonding) molecular orbital and one higher-energy (antibonding) orbital result.
The distribution of electrons in these molecular orbitals is done as per the aufbau principle. Lower-energy orbitals fill first; electrons spread out among degenerate orbitals before pairing, and each orbital can hold a maximum of two electrons with opposite spins.
Bond Order
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order. In the molecular orbital model, an electron contributes to a bonding interaction if it occupies a bonding orbital, and it contributes to an antibonding interaction if it occupies an antibonding orbital. The bond order is calculated by subtracting the destabilizing (antibonding) electrons from the stabilizing (bonding) electrons. Since a bond consists of two electrons, we divide by two to get the bond order. The equation for determining the bond order is as follows:
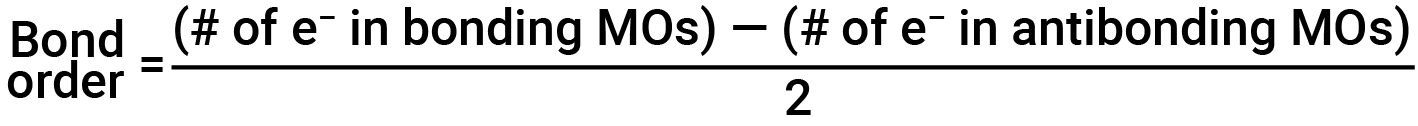
The bond order is a guide to the strength of a covalent bond; a bond between two given atoms becomes stronger as the bond order increases. If the distribution of electrons in the molecular orbitals between two atoms is such that the resulting bond would have a bond order of zero, a stable bond does not form.
Bonding in Homonuclear Diatomic Molecules
A hydrogen molecule (H2) forms from two hydrogen atoms. When the atomic orbitals of the two atoms combine, the electrons occupy the molecular orbital of lowest energy, the σ1s bonding orbital. A dihydrogen molecule, H2, readily forms because the energy of a H2 molecule is lower than that of two H atoms. Both electrons in the H2 molecule are in the σ1s bonding orbital; the electron configuration is (σ1s)2. This configuration is represented by a molecular orbital energy diagram in which a single upward arrow indicates one electron in an orbital, and two (upward and downward) arrows indicate two electrons of opposite spin. A dihydrogen molecule contains two bonding electrons and no antibonding electrons, so the bond order is equal to 1. Thus, the H–H bond is a single bond.
A helium atom has two electrons, both of which are in its 1s orbital. Two helium atoms do not combine to form a dihelium molecule, He2, with four electrons, because the stabilizing effect of the two electrons in the lower-energy bonding orbital would be offset by the destabilizing effect of the two electrons in the higher-energy antibonding molecular orbital. The hypothetical electron configuration of He2 is (σ1s)2(σ*1s)2. The bond order in a hypothetical dihelium molecule would be zero. This indicates that no bond is formed between the two helium atoms.
Bonding in Homonuclear Diatomic Molecules of the Second Period
Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, O2, F2, and Ne2. The Be2 molecule and the Ne2 molecule would not be stable due to zero bond order.
For the valence molecular orbital electron configurations, the valence electrons are assigned to valence molecular orbitals with the lowest possible energies. Consistent with Hund’s rule, whenever there are two or more degenerate molecular orbitals, electrons fill each orbital of that type singly before any pairing of electrons takes place.
σ orbitals are usually more stable than π orbitals. However, this is not always the case. For atoms with three or fewer electrons in the p orbitals (Li through N), a different pattern is observed, in which the σp orbital is higher in energy than the πp set.
This switch in orbital ordering occurs because of a phenomenon called s–p mixing. s–p mixing does not create new orbitals; it merely influences the energies of the existing molecular orbitals. The σs wavefunction mathematically combines with the σp wavefunction, with the result that the σs orbital becomes more stable, and the σp orbital becomes less stable. Similarly, the antibonding orbitals also undergo s–p mixing, with the σs* becoming more stable and the σp* becoming less stable.
s–p mixing occurs when the s and p orbitals have similar energies. The energy difference between 2s and 2p orbitals in O, F, and Ne is greater than that in Li, Be, B, C, and N. Because of this, O2, F2, and Ne2 exhibit negligible s–p mixing (not sufficient to change the energy ordering), and their MO diagrams follow the normal pattern, as shown in the figure above. All of the other period 2 diatomic molecules do have s–p mixing, which leads to the pattern where the σp orbital is raised above the πp set.
This text is adapted from Openstax, Chemistry 2e, Section 8.4: Molecular Orbital Theory.
