19.3:
Nuclear Stability
19.3:
Nuclear Stability
Protons and neutrons, collectively called nucleons, are packed together tightly in a nucleus. With a radius of about 10−15 meters, a nucleus is quite small compared to the radius of the entire atom, which is about 10−10 meters. Nuclei are extremely dense compared to bulk matter, averaging 1.8 × 1014 grams per cubic centimeter. If the earth’s density were equal to the average nuclear density, the earth’s radius would be only about 200 meters.
To hold positively charged protons together in the very small volume of a nucleus requires very strong attractive forces because the positively charged protons repel one another strongly at such short distances. The force of attraction that holds the nucleus together is the strong nuclear force. This force acts between protons, between neutrons, and between protons and neutrons. It is very different from the electrostatic force that holds negatively charged electrons around a positively charged nucleus. Over distances less than 10−15 meters and within the nucleus, the strong nuclear force is much stronger than electrostatic repulsions between protons; over larger distances and outside the nucleus, it is essentially nonexistent.
A plot of the number of neutrons versus the number of protons for stable nuclei reveals that the stable isotopes fall into a narrow band. This region is known as the band of stability (also called the belt, zone, or valley of stability). The straight line in Figure 1 represents nuclei that have a 1:1 ratio of protons to neutrons (n:p ratio). Note that the lighter stable nuclei, in general, have equal numbers of protons and neutrons. For example, nitrogen-14 has seven protons and seven neutrons. Heavier stable nuclei, however, have increasingly more neutrons than protons. For example: the stable nuclide iron-56 has 30 neutrons and 26 protons, an n:p ratio of 1.15, whereas the stable nuclide lead-207 has 125 neutrons and 82 protons, an n:p ratio equal to 1.52. This is because larger nuclei have more proton–proton repulsions and require larger numbers of neutrons to provide compensating strong forces to overcome these electrostatic repulsions and hold the nucleus together.
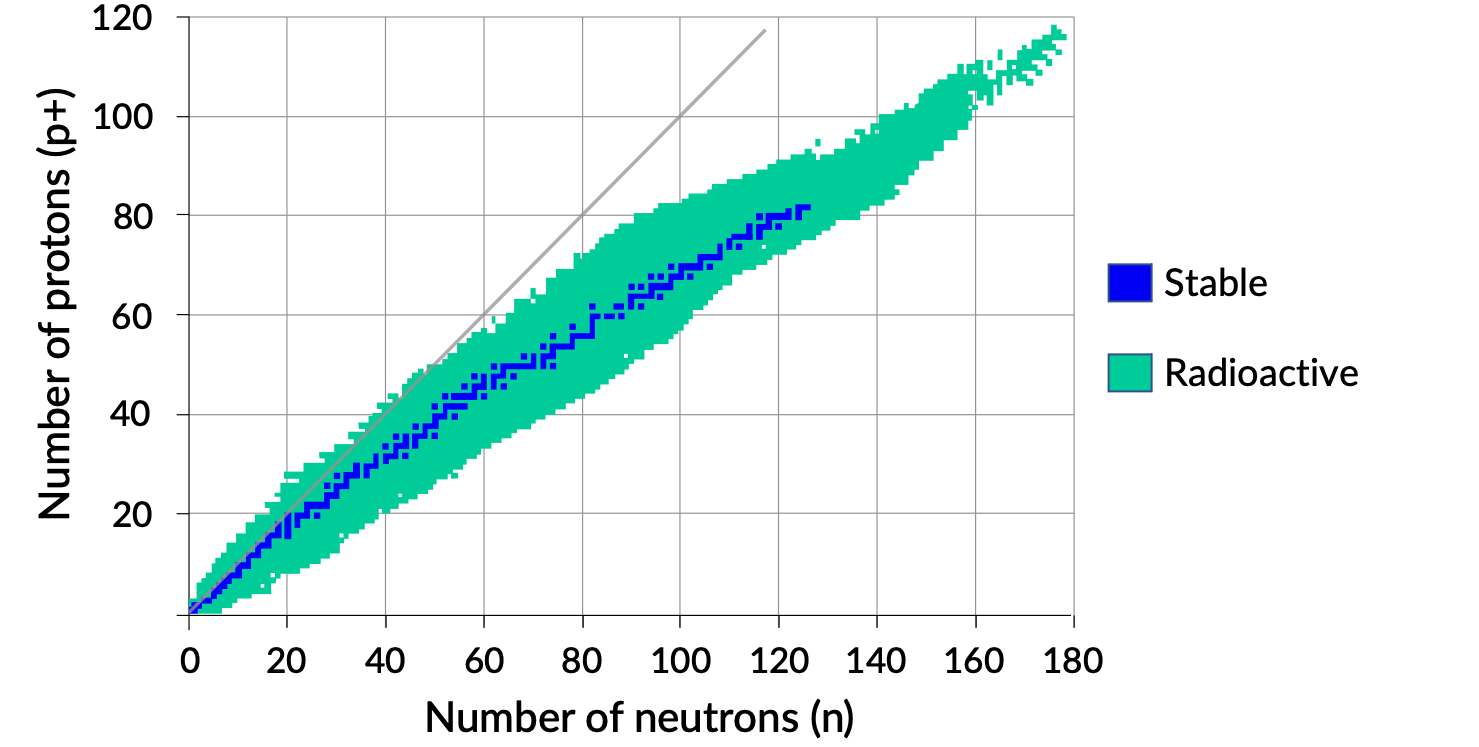
Figure 1. Band of stability.
The nuclei outside the band of stability are unstable and exhibit radioactivity: they change spontaneously, or decay, into other nuclei that are either in or closer to the band of stability. These nuclear decay reactions convert one unstable nuclide, or radionuclide, into another nuclide, which is often more stable.
Several observations may be made regarding the relationship between the stability of a nucleus and its structure.
Nuclei with even numbers of protons, neutrons, or both are more likely to be stable. Nuclei with certain numbers of nucleons, known as magic numbers, are stable against nuclear decay. These numbers of protons or neutrons (2, 8, 20, 28, 50, 82, and 126) make complete shells in the nucleus. These are similar in concept to the stable electron shells observed for the noble gases. Nuclei that have magic numbers of both protons and neutrons are called “doubly magic” and are particularly stable.
Nuclei with atomic numbers higher than 82 are radioactive. Bismuth-209, atomic number 83, was thought to be stable for a very long time and can be handled as though it was non-radioactive. Although it is radioactive, it has an exceptionally long half-life among radionuclides.
The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species in one chain constitute a radioactive family, or radioactive decay series. Three of these series include most of the naturally radioactive elements of the periodic table. They are the uranium series, the actinide series, and the thorium series. The neptunium series is a fourth series, which is no longer significant on the earth because of the short half-lives of the species involved.
This text is adapted from Openstax, Chemistry 2e, Section 21.1: Nuclear Structure and Stability.
