2.1:
Chemical Reactions
Consider a balanced chemical reaction, such as the combustion of hydrogen gas. Here, the quantitative relationship between the reactants and products — H2, O2, and H2O — is that two molecules of H2 react with one molecule of O2 to produce two molecules of H2O. This quantitative relationship is known as stoichiometry.
In a chemical reaction, reactants interact with each other to form products. The reactant that is completely consumed is the limiting reactant, and the reactant present in a quantity greater than necessary to react completely with the limiting reactant is the excess reactant.
A chemical reaction involves the chemical transformation of reactants into products. As the reaction progresses, the concentration of reactants decreases while the concentration of products increases. This variance in concentrations of reactants and products can be plotted in a graph as a function of time.
The speed at which a reaction progresses is called the reaction rate. It measures the rate of reactants’ disappearance or the rate of products’ appearance and is expressed in units of molarity-per-second.
A reversible chemical reaction is one in which the conversion of reactants into products and the conversion of products back into reactants occur simultaneously. A double arrow between the reactants and products signifies its reversible nature.
When the rate of formation of products, or the rate of the forward reaction, equals the rate of formation of reactants, or the rate of the reverse reaction, chemical equilibrium is achieved.
The equilibrium constant expression is written as the molar concentrations of the products, C and D, over the reactants, A and B, at equilibrium, each raised to their respective stoichiometric coefficients. When solved, the expression is equal to the equilibrium constant, Kc.
An expression in the same form can also be written for the reactants and products at any concentration, and the calculated quantity is known as the reaction quotient, Qc.
2.1:
Chemical Reactions
A balanced chemical equation provides the information of chemical formulas of the reactants and products involved in the chemical change. A reaction’s stoichiometry helps predict how much of the reactant is needed to produce the desired amount of product, or in some cases, how much product will be formed from a specific amount of the reactant.
The relative amounts of reactants and products represented in a balanced chemical equation are often referred to as stoichiometric amounts. However, in reality, the reactants are not always present in the stoichiometric amounts indicated by the balanced equation.
In a chemical reaction, the reactant which gets consumed first, and limits the amount of product formed, is the limiting reactant, while the other substance becomes the excess reactant. An excess of one or more reactants is often used to ensure the complete conversion of the other reactant into the product.
Consider the reaction for the formation of water represented by the equation:
Stoichiometry indicates that two moles of hydrogen and one mole of oxygen react to produce two moles of water; that is, hydrogen and oxygen combine in a 2:1 ratio.
Imagine if 5 moles of hydrogen and 2 moles of oxygen are present. The ratio of the reactants is now 5:2 (or 2.5:1), which is greater than the stoichiometric ratio of 2:1. Hydrogen, therefore, is present in excess, and oxygen is the limiting reactant. Reaction of all the provided oxygen (2 mol) will consume 4 mol of the 5 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted. Computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation is one way of identifying the limiting and excess reactants.
The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time dependence of some property that can be related to reactant or product amounts.
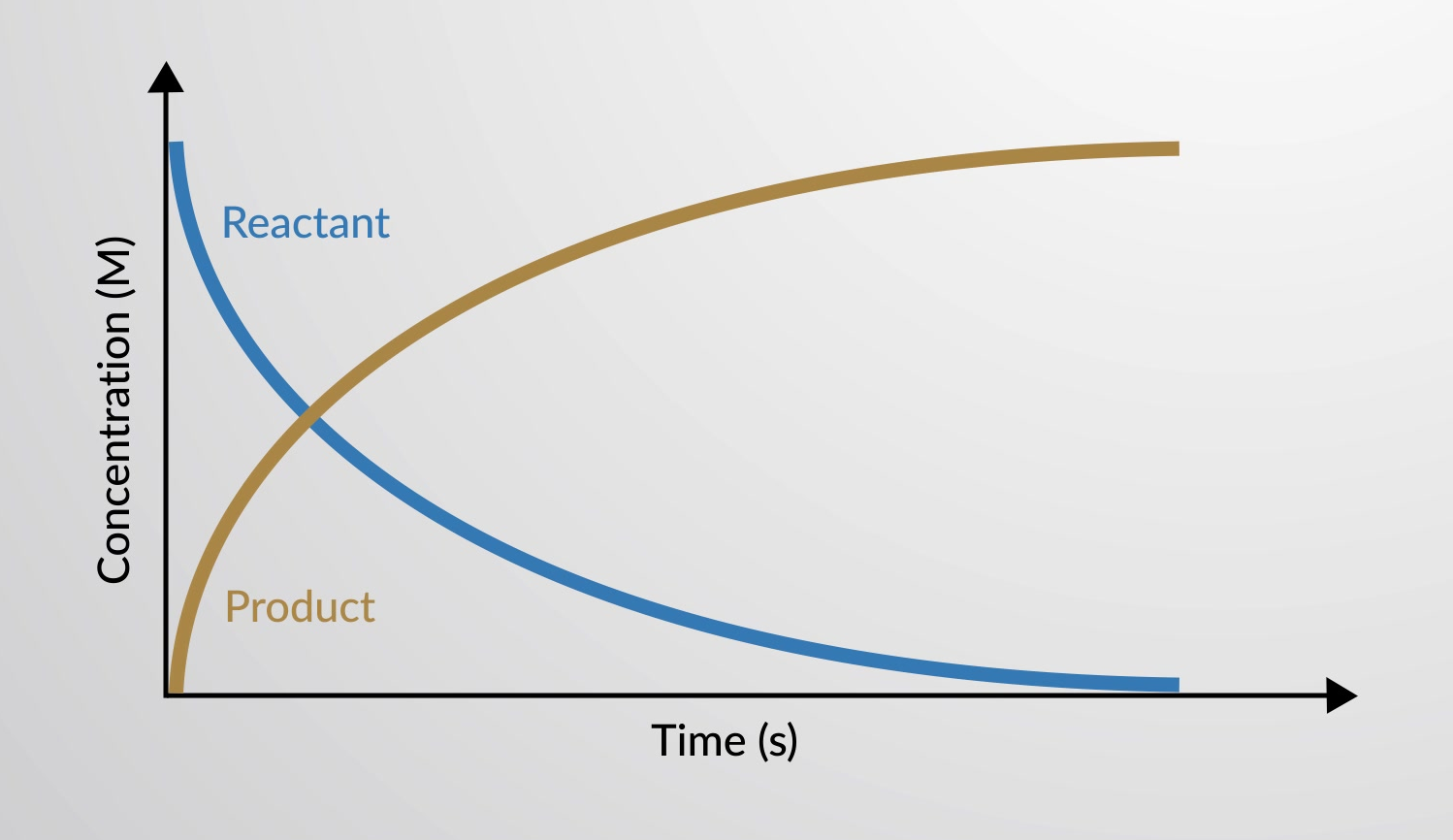
The rate of a chemical reaction can be plotted in a graph as the variance in concentrations of reactants and products as a function of time.
A reversible chemical reaction represents a chemical process that proceeds in both forward (left to right) and reverse (right to left) directions. The status of a reversible reaction is conveniently assessed by evaluating its reaction quotient (Q). For a reversible reaction described by
the reaction quotient is derived directly from the stoichiometry of the balanced equation as
where the subscript c denotes the use of molar concentrations in the expression.
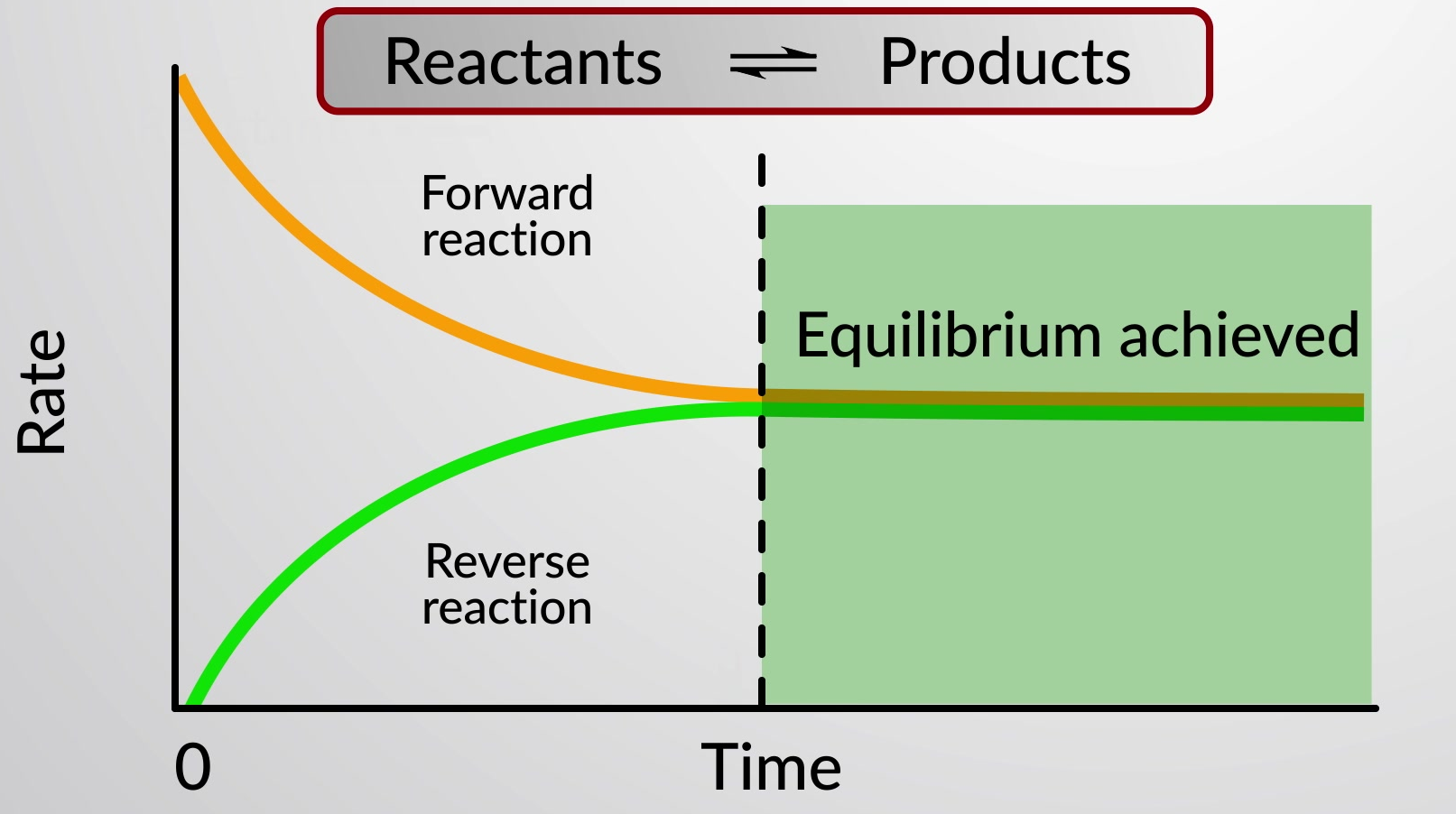
When the rates of the forward and reverse reactions are equal, the concentrations of the reactant and product species remain constant over time and the system is at equilibrium. A special double arrow is used to emphasize the reversible nature of the reaction.
This text is adapted from OpenStax Chemistry 2e, Section 4.3: Reaction Stoichiometry; Section 4.4: Reaction Yield; Section 12.1: Chemical Reaction Rates; Section 13.1 Chemical Equilibria, Section 13.2 Equilibrium Constants.
