5.3:
Acid and Bases: Ka, pKa, and Relative Strengths
An acid protonates a water molecule to form a hydronium ion and its conjugate base. In the reverse reaction, the conjugate base accepts a proton from the hydronium ion. The extent to which an acid dissociates in water defines its strength.
In effect, strong acids completely dissociate in water, and the equilibrium lies on the side of the products, meaning that their solutions contain a high concentration of hydronium ions.
In comparison, weak acids only partially dissociate in water, so the equilibrium favors the reactants. Therefore, their solutions mostly contain undissociated acid molecules with only a few hydronium ions.
The degree of dissociation of a weak acid can be measured using the equilibrium constant, Keq.
In a dilute acid solution, because the change in the concentration of water is negligible, its value essentially remains constant.
Thus, a new equilibrium constant — called the acidity constant, Ka— is defined. Note that the expression has the concentration of hydronium ions in the numerator.
A higher Ka corresponds to a larger hydronium ion concentration, thereby indicating a stronger acid.
The values of Ka range several orders of magnitude for various organic acids. Hence, pKa, expressed as the negative logarithm of Ka, is normally used to indicate the strengths of different acids.
The minus sign in the expression implies that the higher the pKa, the smaller the Ka, and hence the weaker the acid. Therefore, benzoic acid with a pKa of 4.2 is weaker than hydrobromic acid with a pKa of −9.
Additionally, a pKa value can also be used to determine the strength of a base, since every base has a conjugate acid associated with it. The stronger the conjugate acid, the weaker its base.
For example, the pKa of the conjugate acid of methanol is −2.5, which is less than the pKa of the conjugate acid of methylamine.
Consequently, the conjugate acid of methanol is stronger than the conjugate acid of methylamine, and methanol is a weaker base than methylamine.
5.3:
Acid and Bases: Ka, pKa, and Relative Strengths
This lesson delves into a critical aspect of the relative strengths of acids and bases. The strength of an acid is evaluated by the acid dissociation into its conjugate base and a hydronium ion in water. The complete dissociation of a strong acid is confirmed with a very high concentration of hydronium ions. As a result, an incomplete dissociation process affirms a weak acid. Therefore, the equilibrium is in the forward direction for strong acids and backward for weak acids in these reactions.
Accordingly, the acid strength is defined by the concentration of undissociated acid molecules and hydronium ions. While the weak acid can be estimated via the equilibrium constant (Keq), it is constant for a dilute solution, and the change in water concentration is negligible. This observation leads to a modified equilibrium constant known as the acidity constant or dissociation constant, Ka. To define the acidity constant that is a scale of acidity, consider the generic acid-base reaction:
Figure 1: Dissociation of a generic acid in water
Here, HA denotes the generic acid, and A− denotes its conjugate base. The following expression represents the acidity constant for this reaction.
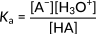
Figure 2: Acidity constant for a generic acid dissociation
This relationship focuses on the concentration of hydronium ions in the numerator. Accordingly, an increase in these ions leads to an increasing acidity constant and a stronger acid. In organic acids, typically, the magnitude of Ka is spread across several orders. Hence, the strength of different acids is expressed in terms of pKa values, calculated as the negative logarithm of Ka:
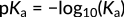
Figure 3: Expression of pKa
Here, the minus sign indicates the inverse relationship between the pKa value and acidity. As elucidated with benzoic acid versus hydrobromic acid, a higher pKa value equals a lower Ka value, which indicates a weaker acid.
By extension of the above principle, the pKa values can also establish the strength of a base. Since the dissociation of a base forms a conjugate acid, a stronger conjugate acid corresponds to a weaker base. For instance, consider methanol versus ethylamine. The conjugate acid of methanol with a pKa value of −3.8 is more acidic than the conjugate acid of ethylamine with a pKa value of 10.6. Hence, methanol is a weaker base than ethylamine.
Suggested Reading
- Brown, W.H., & Iverson, B.L., & Anslyn, V.E., & Foote S.C. (2014). Organic Chemistry. Mason, Ohio: Cengage Learning, 118-123.
- Solomons, G., & Fryhle, C. & Snyder, S. (2015). Organic Chemistry. New Jersey, NJ: Wiley, 192-194.
- Klein, D. (2017). Organic Chemistry. New Jersey, NJ: Wiley, 183-188.
- Clayden, J., & Greeves, N., & Warren, S. (2012). Organic Chemistry. Oxford: Oxford University Press, 300-305.
