9.13:
Bond Energies and Bond Lengths
Every chemical reaction is associated with a change in enthalpy, which helps to determine whether energy is released or required during the reaction. This enthalpy change can be estimated using average bond energies.
The energy required to break a specific bond in 1 mole of a gaseous chemical compound is called bond energy and is expressed in kJ/mol. This energy depends on the type of bonded atoms and the number of shared pairs of electrons.
Bond energy is usually expressed as an average of bond energies of the same bond in different compounds. The average bond energy can be used to determine whether a chemical reaction is exothermic or endothermic.
Consider ethene and bromine reacting to form 1,2-dibromoethane. Initially, the carbon double bond and the bromine single bond break — a process requiring energy input, which increases the potential energy of atoms. Therefore, bond-breaking is an endothermic process with a positive change in enthalpy.
Subsequently, new bonds are formed between carbon and bromine atoms yielding the product. Bond formation increases the stability of the molecule by reducing the potential energy. Therefore, bond formation is an exothermic process, causing a negative change in enthalpy.
According to Hess’s law, the sum of enthalpy changes of reactants and products is equal to the overall enthalpy change of the reaction. The enthalpy change of reactants is the sum of enthalpy of bonds broken, whereas the enthalpy change of products is the sum of enthalpy of new bonds formed.
Therefore, the formation of 1,2-dibromoethane, with an enthalpy of +255 kJ/mol, is an endothermic reaction.
In addition to bond enthalpies, the type of bonds and bonded atoms also influence the bond length, which is the average distance between the nuclei of two bonded atoms.
Consider the different bonds between two nitrogen atoms and two carbon atoms. Atoms with multiple bonds, such as triple bonds, are held more tightly together, leading to shorter and stronger bonds. Consequently, the molecule is more stable and requires higher energy to dissociate.
Generally, the bond strength is indirectly proportional to the bond length, with some exceptions.
9.13:
Bond Energies and Bond Lengths
Stable molecules exist because covalent bonds hold the atoms together. The strength of a covalent bond is measured by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy — the stronger a bond, the greater the energy required to break it.
The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. The bond energy for a diatomic molecule, DX–Y, is defined as the standard enthalpy change for the endothermic reaction:
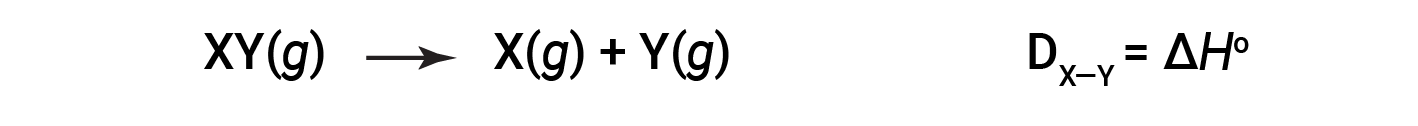
For example, the bond energy of the pure covalent H–H bond, DH–H, is 436 kJ/mol of H–H bonds broken:

Molecules with three or more atoms have two or more bonds. The sum of all bond energies in such a molecule is equal to the standard enthalpy change for the endothermic reaction that breaks all the bonds in the molecule. For example, the sum of the four C–H bond energies in CH4, 1660 kJ, is equal to the standard enthalpy change of the reaction:
The average C–H bond energy, DC–H, is 1660/4 = 415 kJ/mol because there are four moles of C–H bonds broken per mole of the reaction. Although the four C–H bonds are equivalent in the original molecule, they do not each require the same energy to break; once the first bond is broken (which requires 439 kJ/mol), the remaining bonds are easier to break. The 415 kJ/mol value is the average, not the exact value required to break any one bond.
The strength of a bond between two atoms increases as the number of electron pairs in the bond increases. Generally, as the bond strength increases, the bond length decreases. Thus, triple bonds are stronger and shorter than double bonds between the same two atoms; likewise, double bonds are stronger and shorter than single bonds between the same two atoms. When one atom bonds to various atoms in a group, the bond strength typically decreases as we move down the group. For example, C–F is 439 kJ/mol, C–Cl is 330 kJ/mol, and C–Br is 275 kJ/mol.
Bond energies can be used to calculate approximate enthalpy changes for reactions, also called Bond enthalpies, where enthalpies of formation are not available. Calculations of this type will also tell whether a reaction is exothermic or endothermic. An exothermic reaction (ΔH negative, heat produced) results when the bonds in the products are stronger than the bonds in the reactants. An endothermic reaction (ΔH positive, heat absorbed) results when the bonds in the products are weaker than those in the reactants.
The enthalpy change, ΔH, for a chemical reaction is approximately equal to the sum of the energy required to break all bonds in the reactants (energy “in”, positive sign) plus the energy released when all bonds are formed in the products (energy “out,” negative sign). This can be expressed mathematically in the following way:

In this expression, the symbol Ʃ means “the sum of” and D represents the bond energy in kJ/mol, which is always a positive number. The bond energy will depend on whether the particular bond is a single, double, or triple bond. Thus, in calculating enthalpies in this manner, it is important that the bonding in all reactants and products are considered. Because D values are typically averages for one type of bond in many different molecules, this calculation provides a rough estimate, not an exact value, for the enthalpy of reaction.
Consider the following reaction:
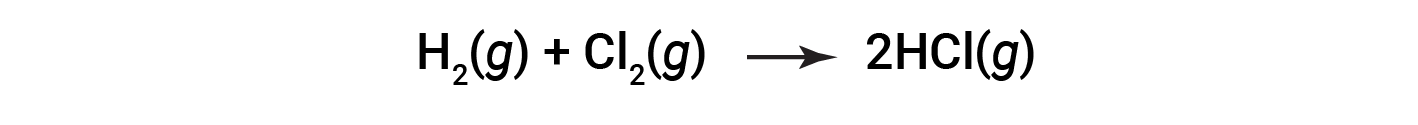
or
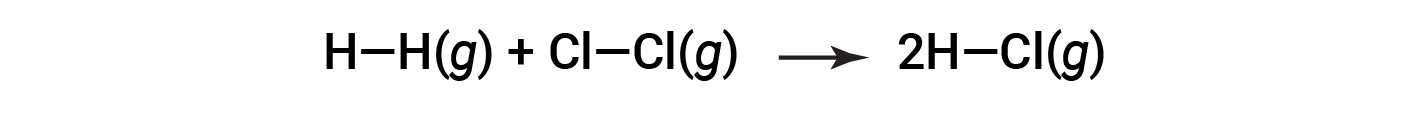
To form two moles of HCl, one mole of H–H bonds and one mole of Cl–Cl bonds must be broken. The energy required to break these bonds is the sum of the bond energy of the H–H bond (436 kJ/mol) and the Cl–Cl bond (243 kJ/mol). During the reaction, two moles of H–Cl bonds are formed (bond energy = 432 kJ/mol), releasing 2 × 432 kJ; or 864 kJ. Because the bonds in the products are stronger than those in the reactants, the reaction releases more energy than it consumes:

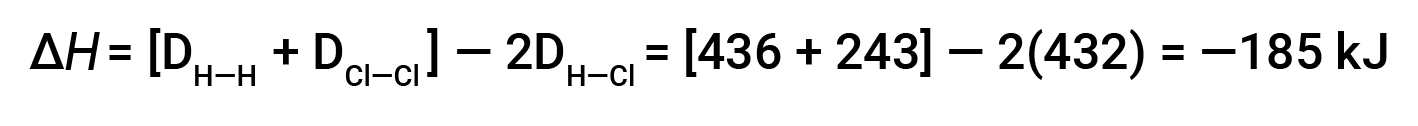
This excess energy is released as heat, so the reaction is exothermic.
This text is adapted from Openstax, Chemistry 2e, Section 7.5: Bond Strength: Covalent Bonds.
