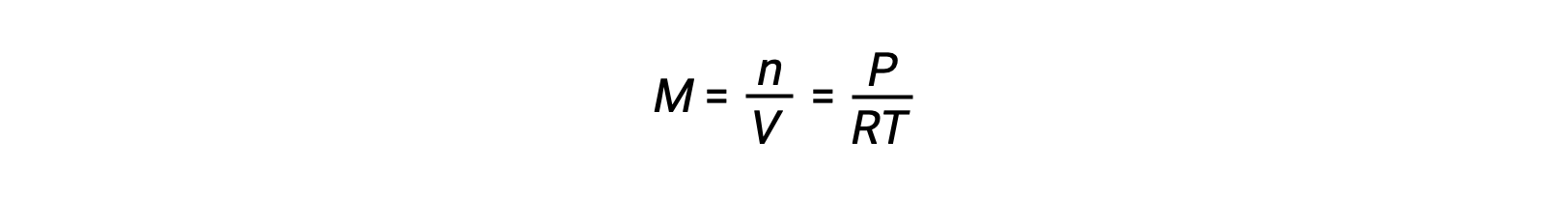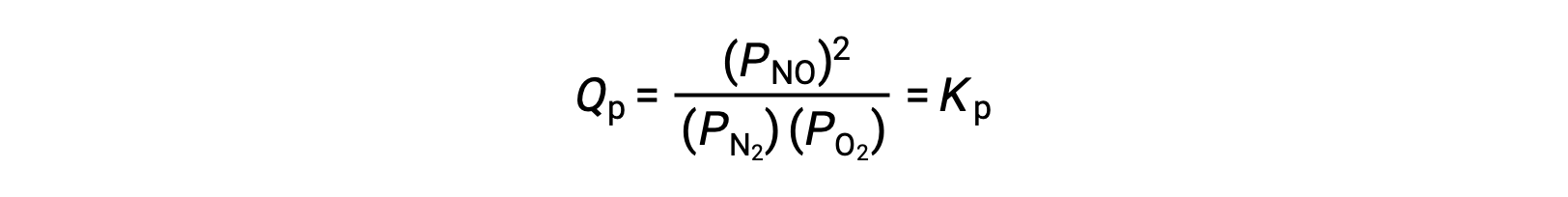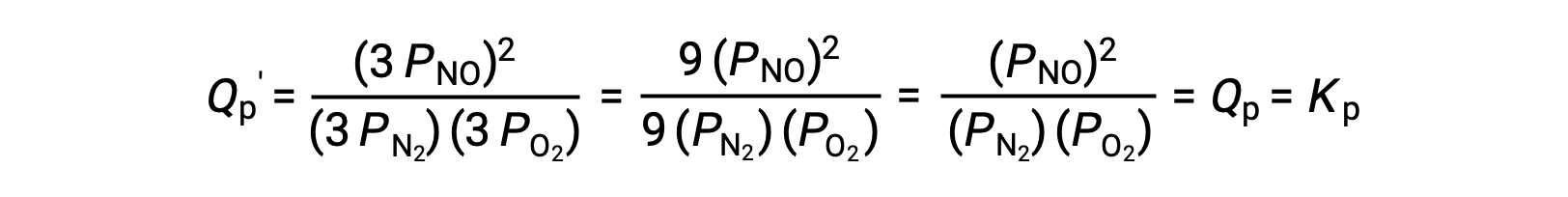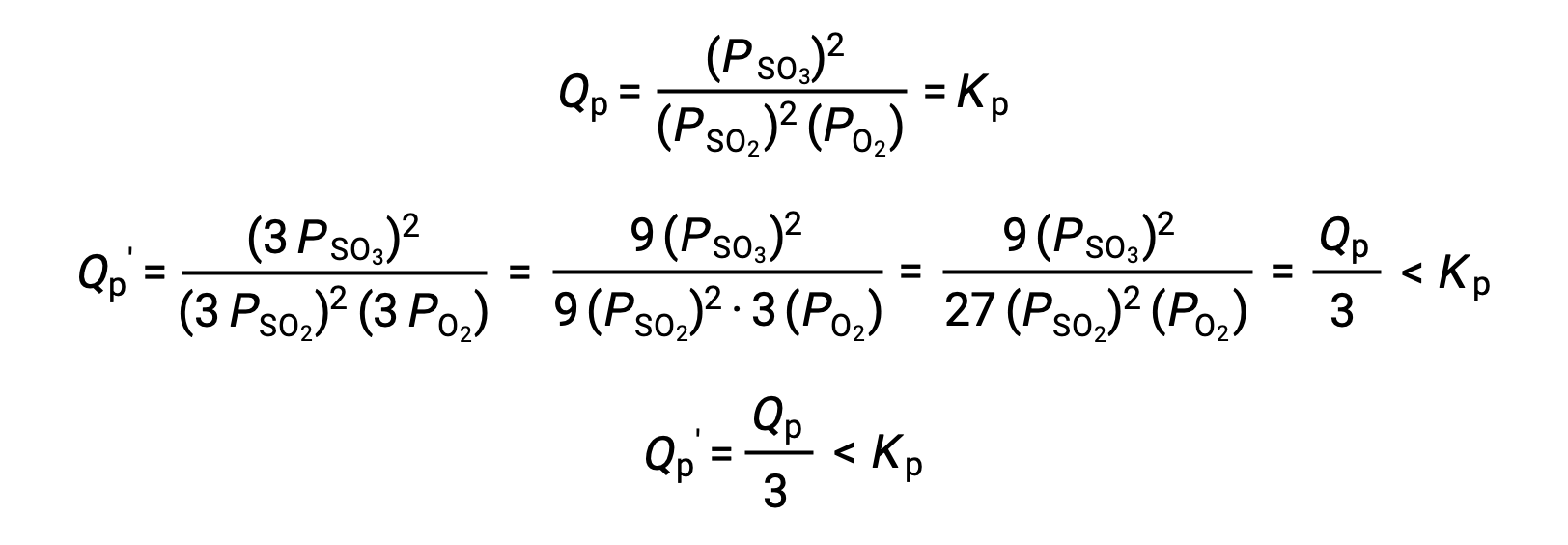Le Châtelier’s principle can be used to predict how a system at equilibrium would respond to the stress of a change in volume or pressure. The volume of a gas is inversely proportional to its pressure; therefore, for a system at equilibrium, a decrease in volume increases the pressure and disturbs the equilibrium. In response, the equilibrium position will shift in a direction to minimize the stress. The ideal gas law states that the pressure of a gas is directly proportional to the number of moles. Thus, the direction of the shift needed to restore equilibrium is dependent on the number of moles of gas particles on either side of the reaction. As more moles of gas results in a higher pressure, an increase in pressure shifts the equilibrium position to the side with fewer moles to lower the pressure. Likewise, a decrease in pressure shifts the equilibrium position to the side with more moles of gas. Consider a chemical equilibrium, where one mole of gaseous phosphorus pentachloride decomposes into one mole of phosphorus trichloride and one mole of chlorine gas—two total moles of product. If the piston is pushed down, the volume of the equilibrium system decreases, increasing the pressure. This disturbs the equilibrium and results in Q greater than K. Thus, the equilibrium position shifts towards the reactants, with fewer moles of gas particles, in order to lower the pressure and restore equilibrium. Conversely, pulling the piston up increases the volume and decreases the pressure. In this case, Q becomes smaller than K. In order to raise the pressure, the equilibrium position shifts towards the products, the side with the most moles of gas, and restores equilibrium. Increasing the pressure by adding an inert gas to an equilibrium mixture at constant volume, does not affect the equilibrium because the partial pressures of the gaseous reactants and products remain unchanged. For equilibrium systems with equal numbers of moles of gaseous reactants and products, such as the reaction between iodine gas and chlorine gas to produce iodine monochloride, a change in the volume of the system will have no effect on the equilibrium.