15.5:
Relative Strengths of Conjugate Acid-Base Pairs
The relationship between the dissociation constants of a conjugate acid-base pair can be expressed quantitatively.
For a weak acid, HA, its acid dissociation constant, Ka, is expressed as the hydronium ion concentration times the A ion concentration divided by the concentration of HA.
The base dissociation constant, Kb, for its conjugate base, A ion, is expressed as the HA concentration times the hydroxide ion concentration divided by the concentration of the A ion.
If the expressions for Ka and Kb are multiplied, the resulting equation is the equilibrium expression for Kw. This equation shows that the Ka for a weak acid and the Kb for its conjugate base are inversely proportional. A stronger acid with a higher Ka always has a proportionately weaker conjugate base with a lower Kb and vice versa.
As the value of KW is constant, this equation can be used to calculate Ka or Kb of a conjugate acid-base pair if either one of them is known. For example, if the Kb for a base is 1 × 10−6, then the Ka for its conjugate acid can be calculated to be 1 × 10−8.
The relationship between Ka, Kb, and KW can also be expressed in terms of their negative logarithms, pKa, pKb, and pKW.
For the equation, Ka times Kb is equal to Kw, when the negative logarithm of both sides is taken, the resulting equation is pKa plus pKb is equal to pKW, which is 14 at 25 °C.
pKa and pKb can also be used to compare the strengths of weak acids and bases. The lower the value of the pKa, the stronger the acid. In the same manner, the lower the value of the pKb, the stronger the base. For example, an acid with a pKa of 2.1 is stronger than an acid with a pKa of 4.6.
15.5:
Relative Strengths of Conjugate Acid-Base Pairs
Brønsted-Lowry acid-base chemistry is the transfer of protons; thus, logic suggests a relation between the relative strengths of conjugate acid-base pairs. The strength of an acid or base is quantified in its ionization constant, Ka or Kb, which represents the extent of the acid or base ionization reaction. For the conjugate acid-base pair HA / A−, the ionization equilibrium equations and ionization constant expressions are
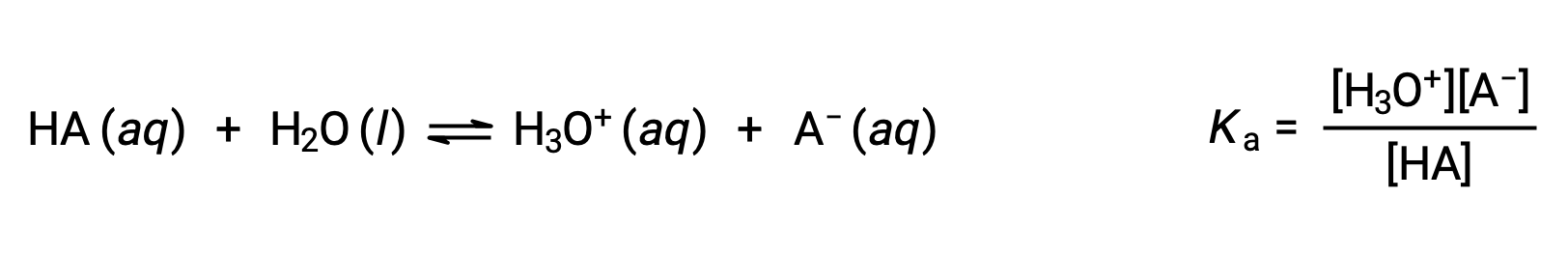
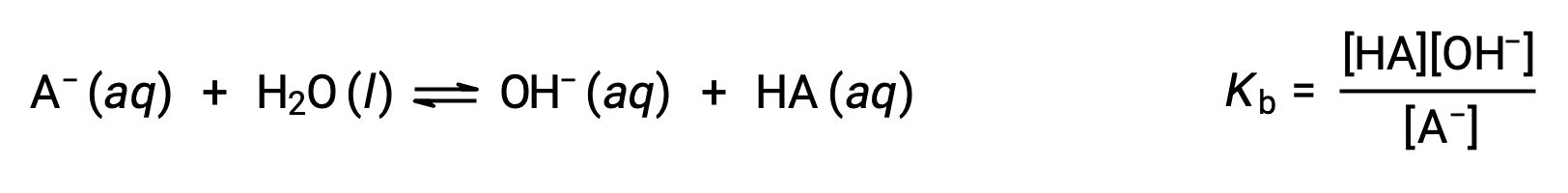
Adding these two chemical equations yields the equation for the autoionization for water:
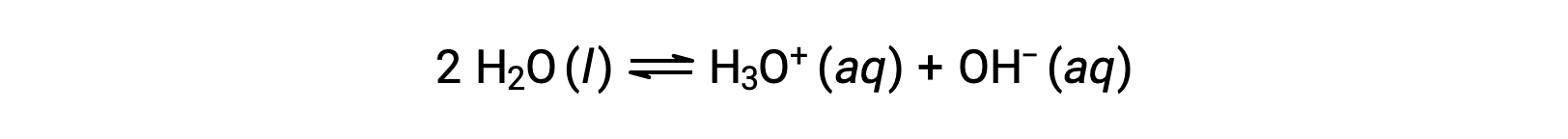
As previously discussed, the equilibrium constant for a summed reaction is equal to the mathematical product of the equilibrium constants for the added reactions, and so
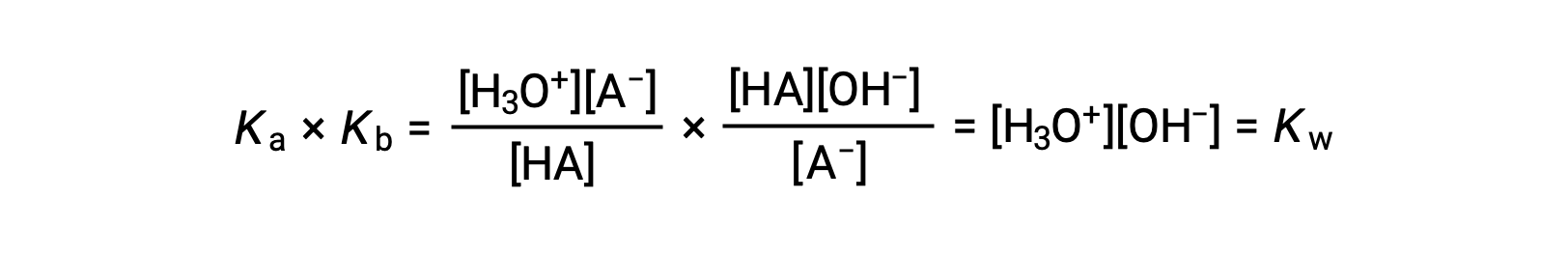
This equation states the relation between ionization constants for any conjugate acid-base pair, namely, their mathematical product is equal to the ion product of water, KW. By rearranging this equation, a reciprocal relation between the strengths of a conjugate acid-base pair becomes evident:
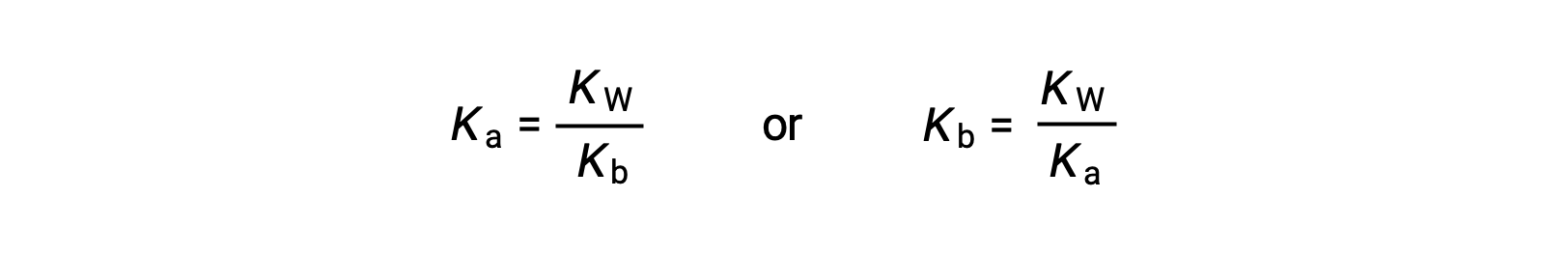
The inverse proportional relation between Ka and Kb means the stronger the acid or base, the weaker its conjugate partner.
Taking the negative log of both sides of the equation, Ka × Kb = KW yields
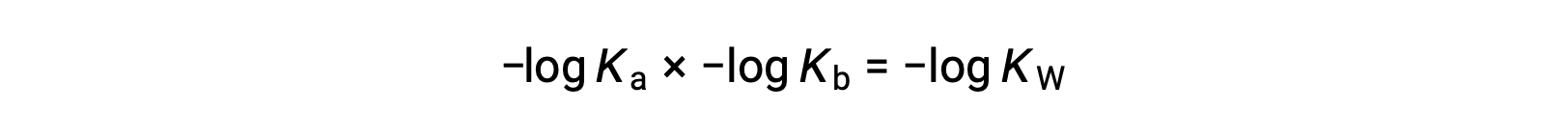
then
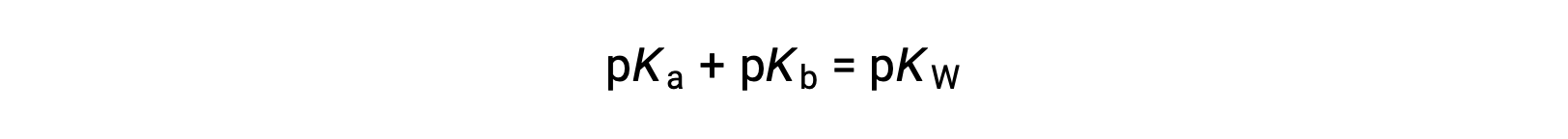
As pKW is 14 at 25 °C, this equation can also be written as
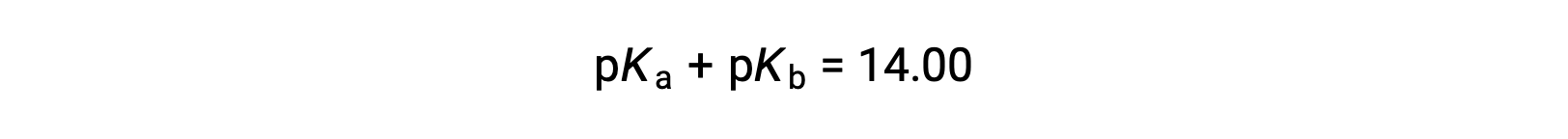
The pKa and pKb also represent the strength of acids and bases, respectively. Like pH and pOH, the higher the value pKa or pKb, the weaker the acid or base, respectively.
| Acid | Base |
| Perchloric acid (HClO4)* | Perchlorate ion (ClO4−)** |
| Sulfuric acid (H2SO4)* | Hydrogen sulfate ion (HSO4−)** |
| Hydrogen iodide (HI)* | Iodide ion (I−)** |
| Hydrogen bromide (HBr)* | Bromide ion (Br−)** |
| Hydrogen chloride (HCl)* | Chloride ion (Cl−)** |
| Nitric acid (HNO3)* | Nitrate ion (NO3−)** |
| Hydronium ion (H3O+) | Water (H2O) |
| Hydrogen sulfate ion (HSO4−) | Sulfate ion (SO42−) |
| Phosphoric acid (H3PO4) | Dihydrogen phosphate ion (H2PO4−) |
| Hydrogen fluoride (HF) | Fluoride ion (F−) |
| Nitrous acid (HNO2) | Nitrite ion (NO2−) |
| Acetic acid (CH3CO2H) | Acetate ion (CH3CO2−) |
| Carbonic acid (H2CO3) | Hydrogen carbonate ion (HCO3−) |
| Hydrogen sulfide (H2S) | Hydrogen sulfide ion (HS−) |
| Ammonium ion (NH4+) | Ammonia (NH3) |
| Hydrogen cyanide (HCN) | Cyanide ion (CN−) |
| Hydrogen carbonate ion (HCO3−) | Carbonate ion (CO32−) |
| Water (H2O) | Hydroxide ion (OH−) |
| Hydrogen sulfide ion (HS−)† | Sulfide ion (S2−)‡ |
| Ethanol (C2H5OH)† | Ethoxide ion (C2H5O−)‡ |
| Ammonia (NH3)† | Amide ion (NH2−)‡ |
| Hydrogen (H2)† | Hydride ion (H−)‡ |
| Methane (CH4)† | Methide ion (CH3−)‡ |
| *Undergo complete acid ionization in water | |
| † Does not undergo acid ionization in water | |
| **Does not undergo base ionization in water | |
| ‡ Undergo complete base ionization in water | |
The listing of conjugate acid–base pairs shown is arranged to show the relative strength of each species as compared with water. In the acid column, those species listed below water are weaker acids than water. These species do not undergo acid ionization in water; they are not Brønsted-Lowry acids. All the species listed above water are stronger acids, transferring protons to water to some extent when dissolved in an aqueous solution to generate hydronium ions. Species above water but below hydronium ion are weak acids, undergoing partial acid ionization, whereas those above hydronium ion are strong acids that are completely ionized in aqueous solution.
This text is adapted from Openstax, Chemistry 2e, Section 14.3: Relative Strengths of Acids and Bases.
