16.3:
Henderson-Hasselbalch Equation
The pH of a buffered solution containing a conjugate acid-base pair may be calculated using the Henderson-Hasselbalch equation as an alternative to an ICE table.
The Henderson-Hasselbalch equation is derived from the equilibrium constant expression for Ka.
This expression can be rearranged to determine the hydronium ion concentration. If the negative log of both sides is taken, the negative logarithm of the hydronium ion concentration and the negative logarithm of the acid dissociation constant can be replaced by the pH and the pKa, respectively.
This yields an equation where the pH of a buffer can be calculated by adding the pKa and the log of the equilibrium concentrations of a conjugate base over its weak acid.
These equilibrium values can be replaced by the initial concentrations if the change in the hydronium ion concentration, x, is less than the 5% of the initial concentrations of both the weak acid and the conjugate base.
The Henderson-Hasselbalch equation also shows the ratio of base to acid needed to prepare a buffer at a specific pH.
Similarly, the pH of a solution containing a weak base and its conjugate acid can be determined using this equation by calculating the pKa of the conjugate acid from the pKb using the formula: pKa plus pKb is equal to fourteen.
The pH of a buffer containing 0.15 molar formic acid and 0.18 molar sodium formate can be determined using either the Henderson-Hasselbalch equation or the Ka for formic acid and the ICE table. However, the Henderson-Hasselbalch equation is a quicker method to calculate the pH when a reaction involves a conjugate acid-base pair and the change in hydronium concentration is small.
The pKa is determined by taking the negative logarithm of the Ka for formic acid, which equals 3.75.
When the initial concentrations of formic acid and formate are plugged into the equation, the pH value for the solution equals 3.83.
This pH value can be used to determine the hydronium ion concentration, 1.5 × 10−4. As this value is less than 5% of 0.15 molar formic acid, the approximations needed to use the Henderson-Hasselbalch equation are valid.
16.3:
Henderson-Hasselbalch Equation
The ionization-constant expression for a solution of a weak acid can be written as:
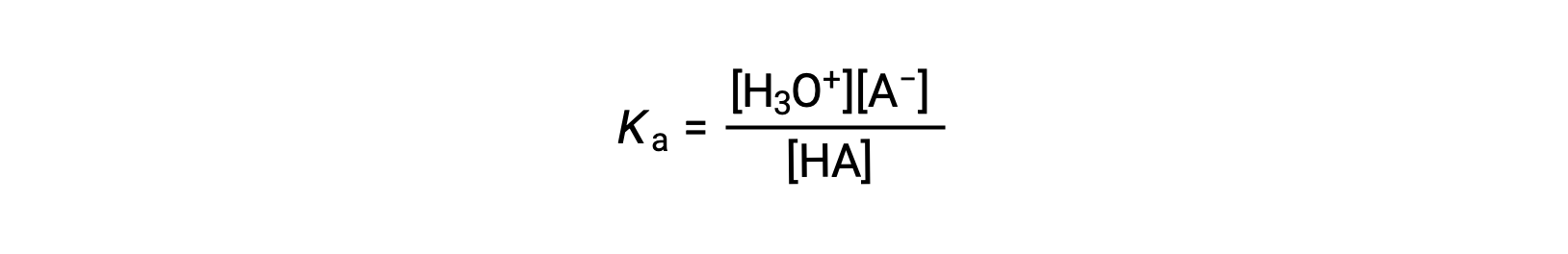
Rearranging to solve for [H3O+] yields:
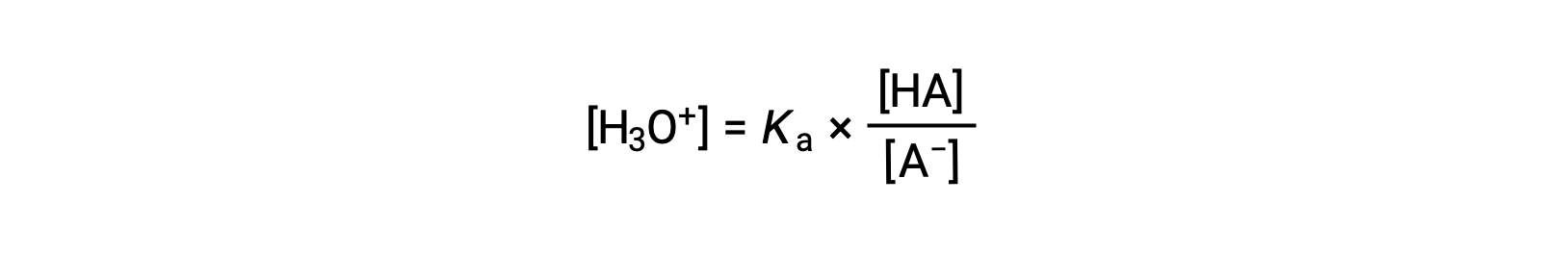
Taking the negative logarithm of both sides of this equation gives
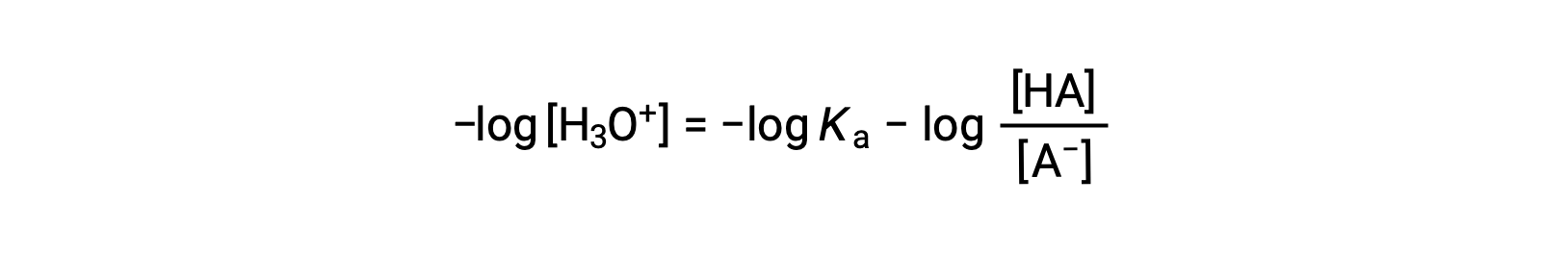
which can be written as
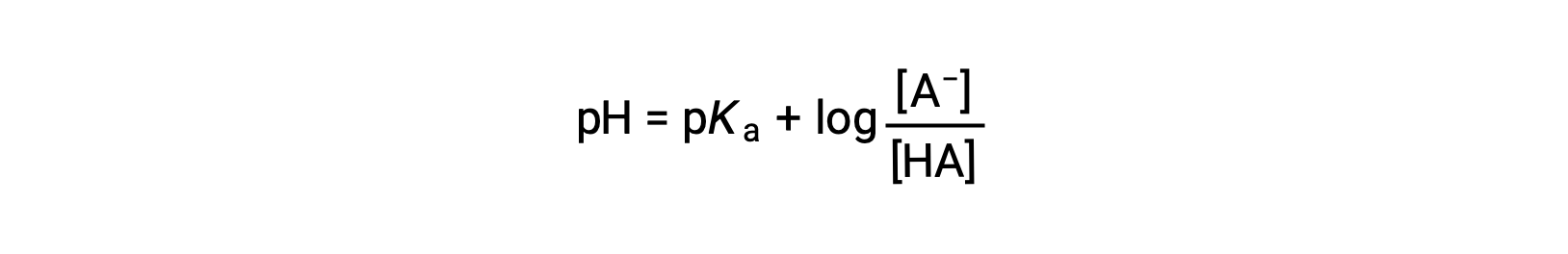
where pKa is the negative of the logarithm of the ionization constant of the weak acid (pKa = −log Ka). This equation relates the pH, the ionization constant of a weak acid, and the concentrations of the weak conjugate acid-base pair in a buffered solution. Scientists often use this expression, called the Henderson-Hasselbalch equation, to calculate the pH of buffer solutions. It is important to note that the “x is small” assumption must be valid to use this equation.
Lawrence Joseph Henderson and Karl Albert Hasselbalch
Lawrence Joseph Henderson (1878–1942) was an American physician, biochemist and physiologist, to name only a few of his many pursuits. He obtained a medical degree from Harvard and then spent 2 years studying in Strasbourg, then a part of Germany, before returning to take a lecturer position at Harvard. He eventually became a professor at Harvard and worked there his entire life. He discovered that the acid-base balance in human blood is regulated by a buffer system formed by the dissolved carbon dioxide in blood. He wrote an equation in 1908 to describe the carbonic acid-carbonate buffer system in blood. Henderson was broadly knowledgeable; in addition to his important research on the physiology of blood, he also wrote on the adaptations of organisms and their fit with their environments, on sociology and on university education. He also founded the Fatigue Laboratory at the Harvard Business School, which examined human physiology with a specific focus on work in industry, exercise, and nutrition.
In 1916, Karl Albert Hasselbalch (1874–1962), a Danish physician and chemist, shared authorship in a paper with Christian Bohr in 1904 that described the Bohr effect, which showed that the ability of hemoglobin in the blood to bind with oxygen was inversely related to the acidity of the blood and the concentration of carbon dioxide. The pH scale was introduced in 1909 by another Dane, Sørensen, and in 1912, Hasselbalch published measurements of the pH of blood. In 1916, Hasselbalch expressed Henderson’s equation in logarithmic terms, consistent with the logarithmic scale of pH, and thus the Henderson-Hasselbalch equation was born.
This text is adapted from Openstax, Chemistry 2e, Section 14.6: Buffers.
Suggested Reading
De Levie, Robert. "The Henderson-Hasselbalch equation: its history and limitations." Journal of Chemical Education 80, no. 2 (2003): 146. https://pubs.acs.org/doi/pdf/10.1021/ed080p146.
