20.9:
Crystal Field Theory – Tetrahedral and Square Planar Complexes
Crystal field theory can be used to model tetrahedral and square planar transition metal complexes in an analogous manner to the application of this theory in octahedral complexes.
For example, to model the tetrahedral tetrachloronickelate(II) ion, each chloride ligand is replaced by a negative point charge, resulting in a tetrahedral crystal field.
Due to the influence of this field, the dxy, dyz, and dxz orbitals are higher in energy than the dx2−y2 and dz2 orbitals. This is attributed to the stronger interaction between the tetrahedral crystal field and the dxy, dyz, and dxz orbitals.
The higher-energy orbitals possess t2 symmetry and are referred to as the t2 set, while the lower-energy orbitals have e symmetry and comprise the e set.
In comparison to the splitting of the d orbitals in octahedral complexes, the relative energies of the orbitals in tetrahedral complexes are inverted and the crystal field splitting energy, or Δtet, is lower.
In square planar complexes such as the tetracyanonickelate(II) ion, all the ligands lie in the xy plane. Here, a square planar crystal field is obtained by replacing the cyanide ligands with negative point charges.
Under the influence of this field, the d orbitals of the metal ion are split into four different energy levels.
Here, the dx2−y2 orbital is the highest-energy orbital and has lobes pointing directly at the ligand charges. The dxy orbital is next in energy with lobes lying in the same plane as the ligand charges.
The dz2 orbital is yet lower in energy, attributed to a small overlap between the dz2 orbital and the crystal field in the xy plane. The lowest energy set of orbitals, dxz and dyz, have relatively minimal interaction with the crystal field.
The crystal field splitting energy in square planar complexes, or Δsp, is defined as the energy difference between the highest-energy orbital, dx2−y2, and the lowest-energy orbitals, dyz and dxz.
Assuming the same metal ion and ligand molecules for all complexes, the ratio of Δtet, Δsp, and Δoct is 0.44:1.7:1.
20.9:
Crystal Field Theory – Tetrahedral and Square Planar Complexes
Tetrahedral Complexes
Crystal field theory (CFT) is applicable to molecules in geometries other than octahedral. In octahedral complexes, the lobes of the dx2−y2 and dz2 orbitals point directly at the ligands. For tetrahedral complexes, the d orbitals remain in place, but with only four ligands located between the axes. None of the orbitals points directly at the tetrahedral ligands. However, the dx2−y2 and dz2 orbitals (along the Cartesian axes) overlap with the ligands less than the dxy, dxz, and dyz orbitals. By analogy with the octahedral case,the energy diagram for the d orbitals in a tetrahedral crystal field can be predicted as shown in Figure 1. To avoid confusion, the octahedral eg set becomes a tetrahedral e set, and the octahedral t2g set becomes a t2 set.
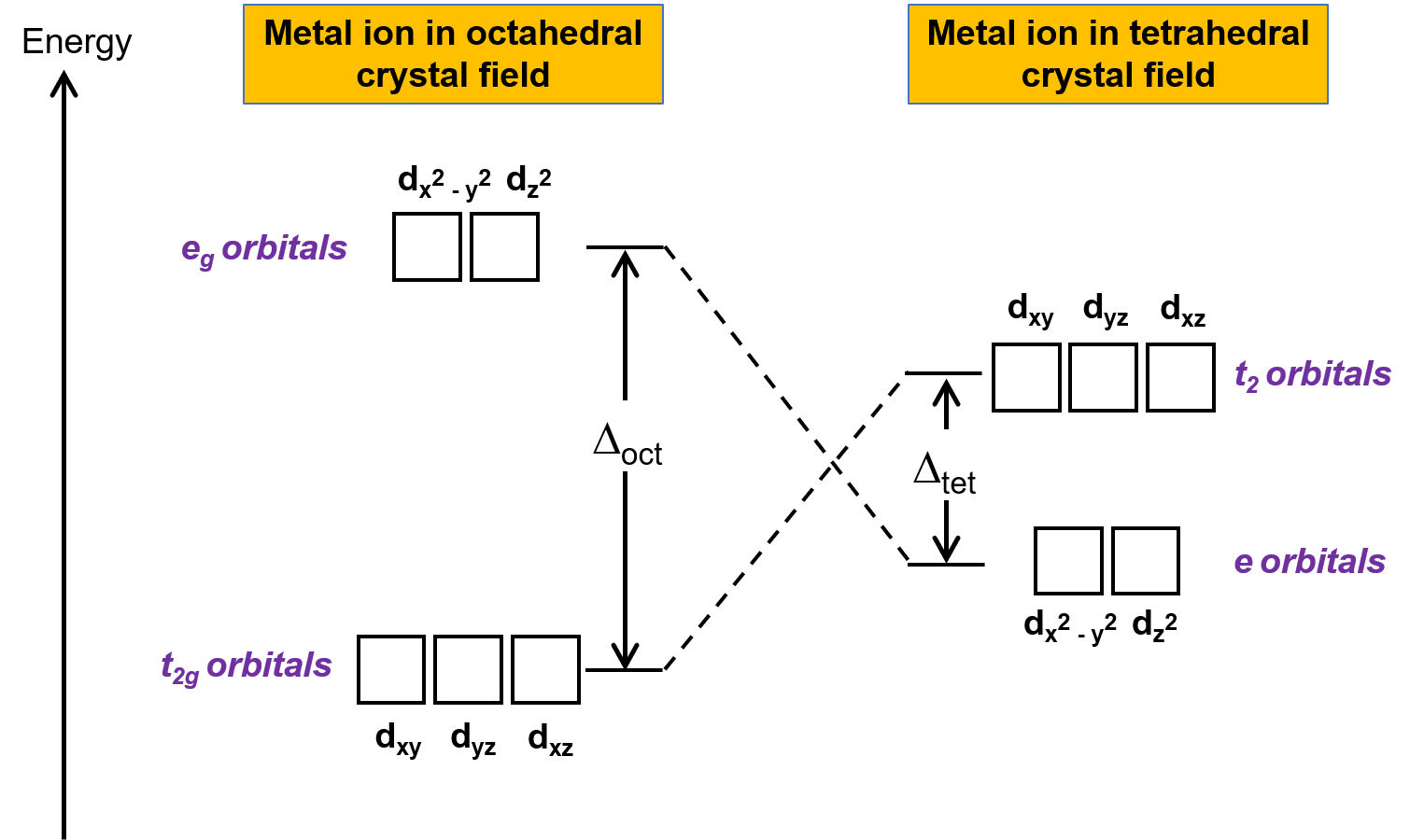
Figure 1. Splitting of the d orbitals of the metal ion under octahedral and tetrahedral crystal fields. In comparison to the octahedral crystal field, the splitting pattern in the tetrahedral crystal field is inverted. The crystal field splitting energy of the octahedral complex, or Δoct, is larger than the crystal field splitting energy of tetrahedral complex, Δtet .
Since CFT is based on electrostatic repulsion, the orbitals closer to the ligands will be destabilized and raised in energy relative to the other set of orbitals. The splitting is less than for octahedral complexes because the overlap is less, so the crystal field splitting energy, or Δtet is usually small.
Square Planar Complexes
The other common geometry is square planar. It is possible to consider a square planar geometry as an octahedral structure with a pair of trans ligands removed. The removed ligands are assumed to be on the z-axis. This changes the distribution of the d orbitals, as orbitals on or near the z-axis become more stable, and those on or near the x- or y-axes become less stable. This results in the octahedral t2g and the eg sets splitting and gives a more complicated splitting pattern (Figure 2).
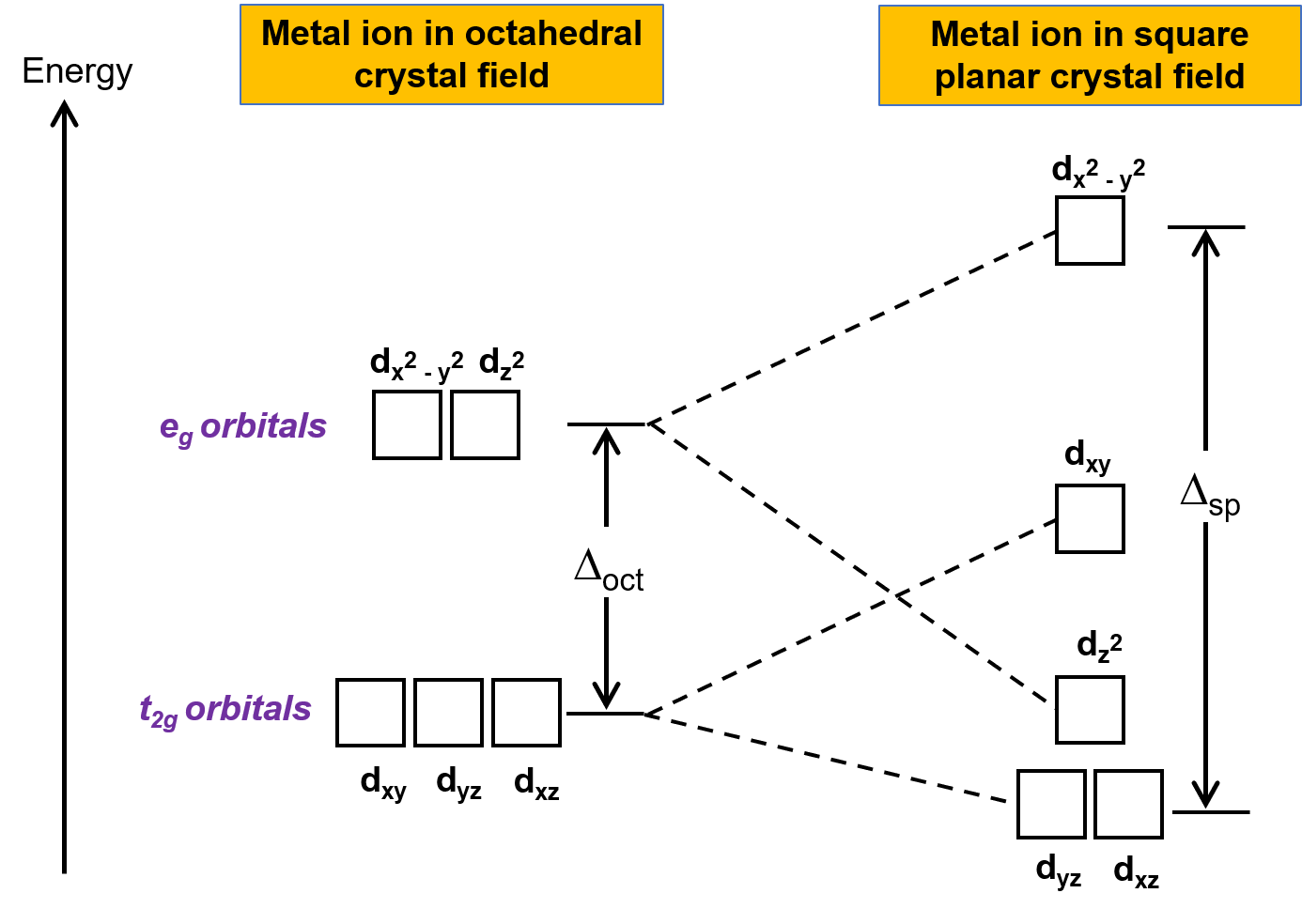
Figure 2. Splitting of the t2g set and the eg set of orbitals in a square planar crystal field. The crystal field splitting energy of square planar complexes, or Δsp, is larger than Δoct.
This text is adapted from Openstax, Chemistry 2e, Section 19.3: Spectroscopic and Magnetic Properties of Coordination Compounds.
