Usando um medidor de pH
English
Share
Overview
Fonte: Laboratório do Dr. Zhongqi He – Departamento de Agricultura dos Estados Unidos
Ácidos e bases são substâncias capazes de doar prótons (H+) e íons hidróxido (OH– ),respectivamente. São dois extremos que descrevem produtos químicos. Misturar ácidos e bases pode cancelar ou neutralizar seus efeitos extremos. Uma substância que não é ácida nem básica é neutra. Os valores de concentração de prótons ([H+]) para a maioria das soluções são inconvenientemente pequenos e difíceis de comparar para que uma quantidade mais prática, pH, tenha sido introduzida. pH foi originalmente definido como o logaritmo decimal da recíproca da concentração molar de prótons, 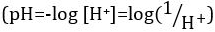 mas foi atualizado para o logaritmo decimal do recíproco da atividade de íons de
mas foi atualizado para o logaritmo decimal do recíproco da atividade de íons de 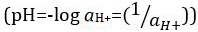 hidrogênio. A primeira definição é agora ocasionalmente expressa como p[H]. A diferença entre p[H] e pH é bastante pequena. Foi declarado que pH = p[H] + 0,04. É prática comum usar o termo ‘pH’ para ambos os tipos de medições.
hidrogênio. A primeira definição é agora ocasionalmente expressa como p[H]. A diferença entre p[H] e pH é bastante pequena. Foi declarado que pH = p[H] + 0,04. É prática comum usar o termo ‘pH’ para ambos os tipos de medições.
A escala de pH normalmente varia de 0 a 14. Para uma solução de 1 M de ácido forte, pH=0 e para uma solução de 1 M de base forte, pH=14. Assim, os valores de pH medidos estarão principalmente na faixa de 0 a 14, embora valores fora dessa faixa sejam inteiramente possíveis. A água pura é neutra com pH=7. Um pH menor que 7 é ácido, e um pH maior que 7 é básico. Como a escala de pH é logarítmica, o pH é uma quantidade inafundada. Cada valor de pH inteiro abaixo de 7 é 10x mais ácido do que o próximo inteiro. Por exemplo, um pH de 4 é 10x mais ácido do que um pH de 5 e 100x (10 x 10) mais ácido do que um pH de 6. O mesmo vale para valores de pH acima de 7, cada um dos quais é 10x mais básico (ou alcalino) do que o próximo valor total mais baixo. Por exemplo, um pH de 10 é 10x mais básico que um pH de 9.
Principles
Procedure
Results
Figure 1 shows the pH of agricultural soils impacted by cropping management and groundwater irrigation. These soil samples were collected from 5 potato fields under different cropping rotation practices with or without groundwater irrigation. The lowest pH is observed in Field 4 soils in both rainfed and groundwater irrigated series. Groundwater irrigation consistently increased soil pH in all 5 fields. The pH information is essential for recommendation of liming the potato fields appropriately to promote optimal growth.

Figure 1. Soil pH of potato fields under different cropping management practices with or without underwater irrigation.
Applications and Summary
pH is one of the most commonly measured chemical parameters of aqueous solutions. It is a critical parameter in water and wastewater treatment for municipal and industrial applications, chemical production, agriculture research, and production. It is also critical in environmental monitoring, chemical and life sciences research, biochemical and pharmaceutical research, electronics production, and many more applications. Figure 2 lists pH values of some common substances.
Pure water is neutral, with a pH of 7.00. When chemicals are mixed with water, the mixture can become either acidic or basic. Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic. Chemicals that are very basic or very acidic are considered "reactive." These chemicals can cause severe burns. Automobile battery acid is an acidic chemical that is reactive. Automobile batteries contain a stronger form of one the acids found in acid rain. Household drain cleaners often contain lye, a very alkaline chemical that is also reactive.
In living systems, the pH of different cellular compartments, body fluids, and organs is usually tightly regulated in a process called acid-base homeostasis. The pH of blood is usually slightly basic with a value of pH 7.365. This value is often referred to as physiological pH in biology and medicine. Plaque can create a local acidic environment that can result in tooth decay by demineralization. Enzymes and other proteins have an optimum pH range and can become inactivated or denatured outside this range.

Figure 2. The pH scale and the pH values of some common items.
Transcript
The pH meter is an electrical device that determines the acidity or basicity of aqueous solutions, one of the most commonly monitored parameters.
To use a pH meter, the pH electrode is first calibrated with standard buffer solutions with known pH values that span the range being measured. To make a pH measurement, the electrode is immersed into the sample solution until a steady reading is reached. The electrode is then rinsed after each sample and stored in a storage solution after all the measurements have been completed.
This video will demonstrate how to calibrate a pH meter and obtain pH measurements, as well as offer a few tips on handling the fragile electrode.
When an acid or a base is placed in water, hydrolysis reactions can occur. The amount of the hydronium ions or the hydroxide ions yielded in the reaction determines the acidity or basicity of the solution. This important property is commonly evaluated by the concentration of the hydronium ion, which is often shortened as hydrogen ion or proton. For most solutions, the hydrogen ion concentration, expressed in moles per liter, is very small, so a more practical quantity, pH, has been introduced.
pH is defined as the negative logarithm of the molar concentration of the hydrogen ion. The pH scale ranges from 0 to 14. Pure water is neutral with a pH of 7; pH less than 7 is acidic, and pH greater than 7 is basic. Since the pH scale is logarithmic, a unit decrease in pH equals a ten-fold increase in acidity.
So how does the pH meter measure pH? A key component of a pH meter is a hydrogen ion-sensitive electrode. The solution inside this electrode contains a known concentration of hydrogen ions. When the electrode is immersed in a solution of unknown pH, an electric potential develops as a function of the hydrogen ion concentration in the test solution. This hydrogen ion-sensitive electrode, along with a reference electrode with which it is often combined into one body, is connected to the pH meter, so that the developed potential can be measured and converted to the pH value.
Now that you understand the theory behind a pH meter, let’s look at its use in an agricultural setting.
Before pH measurements, the pH meter is calibrated. Calibration should be performed at the beginning of each day or before each measurement if extremely precise data are required.
Choose buffers that span the range of pH values of the samples. In this demonstration, the pH meter is calibrated with three buffers with pH 4, 7, and 10. Make sure the buffers are fresh, unused, and unexpired.
To begin, turn on the pH meter by pressing the power button.
Next, plug the pH probe and automatic temperature compensation, or ATC probe, into the unit. On the LCD display, make sure the measurement mode is “pH”. If not, press the “MODE” button until “pH” appears.
Then, remove the pH electrode from the storage buffer. Be careful not to allow the electrode bulb to dry out at any point during the experiment. Rinse the electrode with distilled water, followed by the pH 7 standard buffer.
Next, immerse the pH electrode into the buffer. Stir the buffer with a magnetic bar for best results. To avoid breaking the fragile electrode, be sure to keep a distance between the electrode and the stirring bar.
Press the “CAL(ibration) / MEAS(urement)” button to select the calibration function. Set the buffer pH value to 7.00. When the reading is stable, press “ENTER”. The primary reading flashes briefly; then the secondary display shows the remaining buffers. The electrode is now ready to be calibrated with the next standard buffer.
Rinse the pH electrode as before, first with distilled water, then the pH 4 standard buffer. Then, place the electrode in the buffer. When the reading is stable, press “ENTER”. The primary reading flashes briefly, followed by a display of the percent efficiency, or slope, before the remaining buffers are shown on the secondary display.
Finally, repeat the rinse and calibration steps with the pH 10 standard buffer. The steady reading should be pH 10.01. Once “Enter” is pressed, the 3-point calibration is done, and the meter will automatically return to measurement mode.
The device is now ready to be used to test soil samples from a potato field.
Start pH measurements by thoroughly rinsing the pH electrode with distilled water. Gently blot the electrode on a laboratory cleaning tissue to remove excess water. Be careful not to rub the bulb as it can cause a static charge buildup. The rinse step should be performed between each sample to prevent contamination.
Next, dip the pH electrode into a sample with stirring. The stirring speed should be the same as during calibration. Wait for the reading to become stable, which should take less than 60 s for most samples, then record the pH value. If needed, press the “HOLD” button to freeze the reading display. Press again to resume live reading. The pH value can be stored into memory by pressing the “MI” (or memory insert) button. The stored memory location value, or StO, will be briefly displayed.
Repeat the rinse and measurement steps as previously shown for all the remaining samples. Once all the measurements are completed, thoroughly rinse the electrode before placing it in storage solution.
In this experiment, the pH of multiple soil samples from agricultural fields was measured. pH has numerous effects on crop growth, including nutrient availability, toxicity, and disease control. Different crops have pH ranges of optimal growth. By controlling the pH, disease can be minimized while increasing yield.
The soil samples were collected from five potato fields under different cropping rotation practices with or without groundwater irrigation. Groundwater irrigation consistently increased soil pH in all five fields. These data are essential for providing liming recommendation for the potato fields.
Many fields of science require pH measurements or monitoring in their research.
For example, to use biomass more efficiently and better understand plant cell wall architecture, a series of reactions was carried out to synthesize biomimics of wood, so that plant cell wall architecture can be better understood.
In the first step, kraft pulp fiber was used to generate nanofibrillated cellulose. The pH of the reaction mixture decreased as the hydroxyl groups on the cellulose were oxidized.
The pH was continuously monitored and adjusted by adding sodium hydroxide. Once all the accessible hydroxyl groups were oxidized, the pH would no longer change and the reaction was complete.
In this environmental study, water runoff was analyzed in a facility simulating urban landscapes. Runoff has the potential to carry nutrients and sediments into local streams and lakes where they may contribute to eutrophication.
A facility with multiple field plots was constructed, and runoff water was collected. The pH of the runoff samples, along with other chemical constituents, was quantified.
In life science research, pH is also of great interest as it is strictly regulated in living organisms. In this example, fluorescent pH sensors were developed.
To calibrate these sensors in vitro, a pH titration was performed in a cuvette, where the pH of the sensor solution was measured with a microelectrode, and the emission spectra at each pH were obtained. This way, the fluorescence intensity could be plotted against the pH to generate a calibration curve.
These sensors were then used to measure pH in living cells.
You’ve just watched JoVE’s introduction to using a pH meter. You should now understand what pH is, how the meter works, and how to use one to measure pH.
Thanks for watching!
