4.8:
Chemical Reactions in Aqueous Solutions
Many chemical reactions occur in aqueous solutions. These reactions fall into 3 main categories: precipitation, acid–base, and oxidation–reduction.
In a typical precipitation reaction, dissolved cations and anions come together to form an insoluble ionic compound that precipitates from the solution. For example, the reaction between aqueous potassium chloride and aqueous silver nitrate leads to the precipitation of insoluble silver chloride.
In an acid–base reaction, the acid and base neutralize each other, such as when a proton (H+ ion) from an acid and a OH− ion from a base react to form water. The counterions typically react to form a salt. For example, hydrogen bromide reacts with potassium hydroxide to give water and potassium bromide.
In an oxidation–reduction, or redox, reaction, electrons are transferred from one reactant to another. For example, an atom of potassium loses one electron to an atom of chlorine when they combine to form potassium chloride.
When a chemical reaction occurs in an aqueous solution, water-soluble molecular solids dissolve as intact molecules, whereas water-soluble ionic solids exist as separated ions. Ionic solids that are water-insoluble remain undissolved.
While writing equations for reactions that occur in aqueous solution, the nature of the dissolved substance must be indicated.
Consider the precipitation reaction between aqueous lead(II) nitrate and aqueous sodium iodide to form solid lead(II) iodide and aqueous sodium nitrate.
The balanced equation is called the molecular equation because the complete neutral formulas of the compounds are written as if they existed as molecules or whole units in solution.
Water-soluble ionic compounds such as lead(II) nitrate, sodium iodide, and sodium nitrate exist as ions in solution, whereas water-insoluble lead(II) iodide exists as an ionic solid.
The molecular equation can, therefore, be rewritten as a complete ionic equation, which shows soluble ionic species as free ions in solution.
The ions that appear in identical forms on both sides of the complete ionic equation are called spectator ions. Canceling out spectator ions leaves the net ionic equation, which includes only the ions and molecules that actually participate in the reaction.
4.8:
Chemical Reactions in Aqueous Solutions
Chemical substances interact in many different ways. Certain chemical reactions exhibit common patterns of reactivity. Due to the vast number of chemical reactions, it becomes necessary to classify them based on the observed patterns of interaction.
Water is a good solvent that can dissolve many substances. For this reason, many chemical reactions take place in water. Such reactions are called aqueous reactions. The three most common types of aqueous reactions are precipitation, acid-base, and oxidation-reduction.
Reactions in Aqueous Solutions
A precipitation reaction involves the exchange of ions between ionic compounds in aqueous solution to form an insoluble salt or a precipitate. In an acid-base reaction, an acid reacts with a base, and the two neutralize each other, producing salt and water. An oxidation–reduction reaction involves the transfer of electrons between reacting species. The reactant that loses electrons is said to be oxidized, and the reactant that gains electrons is said to be reduced.
Equations for Aqueous Reactions
When ions are involved, there are various ways of representing the reactions that take place in aqueous media, each with a different level of detail. To understand this, let us take an example of a precipitation reaction. The reaction is between aqueous solutions of ionic compounds, like BaCl2 and AgNO3. The products of the reaction are aqueous Ba(NO3)2 and solid AgCl.

This balanced equation is called a molecular equation. Molecular equations provide stoichiometric information to make quantitative calculations and also helps identify the reagents used and the products formed. However, molecular equations do not provide the details of the reaction process in solution; that is, it does not indicate the different ionic species that are present in solution.
Ionic compounds such as BaCl2, AgNO3, and Ba(NO3)2 are water-soluble. They dissolve by dissociating into their constituent ions, and their ions are homogeneously dispersed in solution.



Since AgCl is an insoluble salt, it does not dissociate into ions and stays in solution as a solid. Considering the above factors, a more realistic representation of the reaction would be:
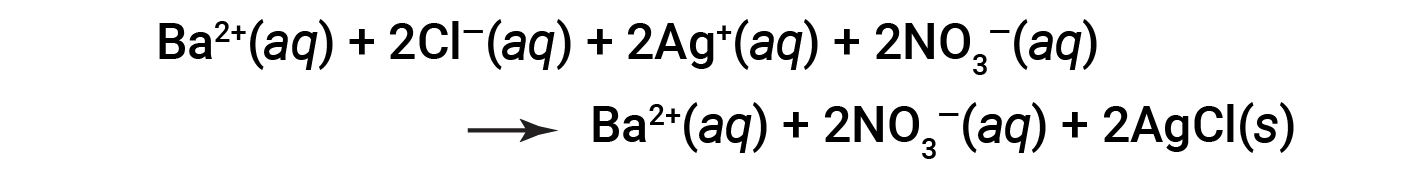
This is the complete ionic equation in which all dissolved ions are explicitly represented.
This complete ionic equation indicates two chemical species that are present in identical form on both sides, Ba2+ (aq) and NO3− (aq). These are called spectator ions. The presence of these ions is required to maintain charge neutrality. Since they are neither chemically nor physically changed by the process, they may be eliminated from the equation.

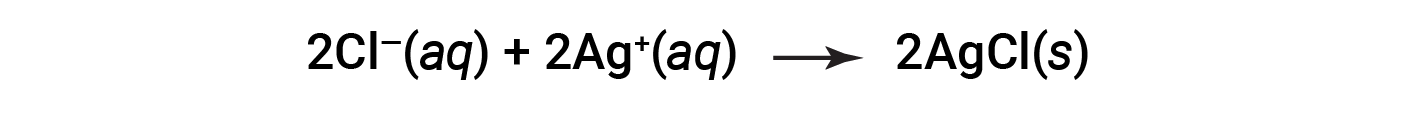
This equation can be further simplified to give:
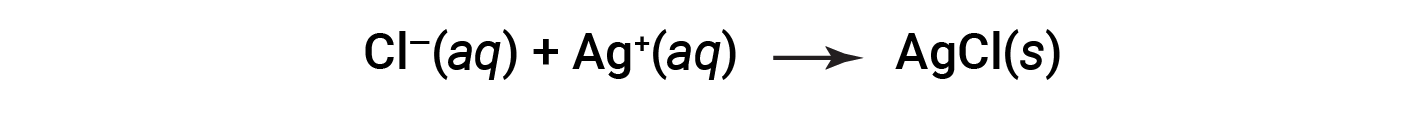
This is the net ionic equation. It indicates that solid silver chloride may be produced from dissolved chloride and silver ions, regardless of the source of these ions.
This text is adapted from OpenStax Chemistry 2e, Section 4.1: Writing and Balancing Chemical Equations.
