6.4:
Quantifying Heat
Internal energy is defined by the interconversion of heat and work. But how is heat measured?
Every substance possesses thermal energy. When a system experiences a temperature difference with its surroundings, thermal energy flows from the matter of higher temperature to the matter of lower temperature. This thermal energy transfer is also called heat transfer and is measured in Joules.
Temperature, on the other hand, describes a property of thermal energy and is measured in Celsius or Kelvin. Thus, the temperature is substance independent, unlike heat, which depends on the number and type of molecules in a system.
Heat can be measured by a technique called calorimetry, which uses the relationship between the heat and a change in temperature of a system.
When a piece of iron at 114 °C is placed into room temperature water, heat flows from the iron to the water until they are the same temperature. Once there is no net flow of heat, they are in thermal equilibrium.
In an isolated system, the water gains exactly as much heat as the iron loses. But the final temperature is only one degree above the water’s starting temperature, while the iron has cooled by 93 °C.
The amount of heat transferred is proportional to the temperature change ΔT.
The proportionality constant C or the “heat capacity”, is the amount of heat needed to increase a system’s temperature by one kelvin, or one degree Celsius.
Heat capacity depends on the type of the substance: a system with a large heat capacity like water needs to absorb more heat to raise its temperature than a system with a smaller heat capacity, such as iron.
Heat capacity also depends on the mass of the substance. Specific heat capacity, Cs, or molar heat capacity, Cm, describes the amount of heat needed to increase the temperature of 1 gram, or 1 mole of a substance by 1 °C. Thus, the unit is given in either Joules per gram degree Celsius or as Joules per mole degree Celsius.
Thus, if specific heat capacities and temperature change of a system are known, the value for heat can be calculated from their product.
6.4:
Quantifying Heat
Thermal Energy
Microscopically, thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Temperature is a quantitative measure of “hot” or “cold”, which depends on the amount of thermal energy. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (KE) (or higher thermal energy), and the object is perceived as “hot”, or it is described as being at a higher temperature. When the atoms and molecules are moving slowly, they have lower average KE (or lower thermal energy), and the object is perceived as “cold”, or it is described as being at a lower temperature.
Assuming that no chemical reaction or phase change (such as melting or vaporizing) occurs, increasing the amount of thermal energy in a sample of matter will cause its temperature to increase, while decreasing the amount of thermal energy in a sample of matter will cause its temperature to decline.
Heat
Heat (q) is the transfer of thermal energy between two bodies at different temperatures. Heat flow increases the thermal energy of one body and decreases the thermal energy of the other. Heat flows spontaneously from hot to cold (i.e., one direction only) and continues until the two substances are at the same temperature. Changes in heat are measured through changes in temperature.
The SI unit of heat, work, and energy is the joule. A joule (J) is defined as the amount of energy used when a force of 1 newton moves an object 1 meter. It is named in honor of the English physicist James Prescott Joule. One joule is equivalent to 1 kg m2/s2, which is also called 1 newton–meter. One kilojoule (kJ) is 1000 joules. To standardize its definition, one calorie has been set to equal 4.184 joules.
Thermal Energy Transfer
The heat capacity (C) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (ΔT) of 1 degree C (or equivalently, 1 kelvin):
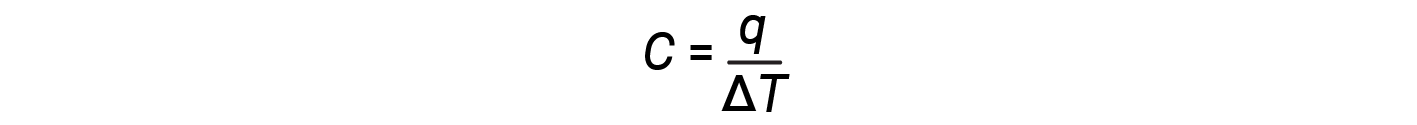
Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. Therefore, heat capacity is an extensive property — its value is proportional to the amount of the substance. For example, consider the heat capacities of two cast iron frying pans. The heat capacity of the large pan is five times greater than that of the small pan because, although both are made of the same material, the mass of the large pan is five times greater than the mass of the small pan. More mass means more atoms are present in the larger pan, so it takes more energy to make all of those atoms vibrate faster. The heat capacity of the small cast iron frying pan is found by observing that it takes 18,140 J of energy (q) to raise the temperature of the pan by 50.0 °C (ΔT):

The larger cast iron frying pan, while made of the same substance, requires 90,700 J of energy (q) to raise its temperature by 50.0 °C (ΔT). The larger pan has a (proportionally) larger heat capacity because the larger amount of material requires a (proportionally) larger amount of energy to yield the same temperature change:

The specific heat capacity (c) of a substance, commonly called its “specific heat,” is the quantity of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius (or 1 kelvin):

Specific heat capacity depends only on the kind of substance absorbing or releasing heat. It is an intensive property—so it is not dependent on the amount of the substance. For example, the small cast iron frying pan has a mass of 808 g. The specific heat of iron (the material used to make the pan) is, therefore:

The large frying pan has a mass of 4040 g. Using the data for this pan, we can also calculate the specific heat of iron:

Although the large pan is more massive than the small pan, since both are made of the same material, they both yield the same value for specific heat (for the material of construction, iron). Note that specific heat is measured in units of energy per temperature per mass and is an intensive property, being derived from a ratio of two extensive properties (heat and mass). The molar heat capacity, also an intensive property, is the heat capacity per mole of a particular substance and has units of J/mol⋅°C.
Water has a relatively high specific heat (about 4.2 J/g⋅°C for the liquid and 2.09 J/g⋅°C for the solid); most metals have much lower specific heats (usually less than 1 J/g⋅°C). Knowing the mass (m) of a substance and its specific heat (c), one can determine the amount of heat, q, entering or leaving the substance by measuring the temperature change (ΔT) before and after the heat is gained or lost:
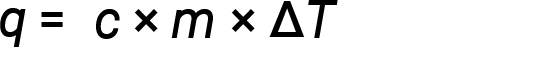
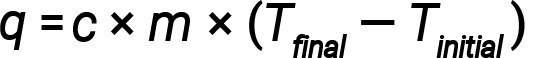
If a substance gains thermal energy, its temperature increases, its final temperature is higher than its initial temperature, Tfinal − Tinitial has a positive value, and the value of q is positive. If a substance loses thermal energy, its temperature decreases, the final temperature is lower than the initial temperature, Tfinal − Tinitial has a negative value, and the value of q is negative.
Note that the relationship between heat, specific heat, mass, and temperature change can be used to determine any of these quantities (not just heat) if the other three are known or can be deduced.
This text is adapted from OpenStax Chemistry 2e, Section 5.1: Energy Basics and OpenStax Chemistry 2e, Section 5.3: Enthalpy.
