6.5:
Quantifying Work
Recall that a system’s internal energy is defined as the sum of heat and work. Heat is measured using calorimetry, but how is work quantified?
Work is the result of a force, which an object experiences – such as by being pulled, pushed, or lifted – over a distance. Thus work equals force times distance.
If a golf club hits a ball 100 feet, then it has done work. Energy is transferred from the club to the ball.
In this case, the ball is the system on which work is performed by the surroundings.
In chemical reactions, work can be associated with multiple physical or chemical changes in a system. A commonly encountered type of work is pressure-volume work.
Consider the combustion in an engine’s cylinder. Not only does the combustion generate heat, it also produces gas, which performs work.
When the gas volume in the cylinder expands, the pressure pushes the piston downward acting against external forces in the surroundings.
Pressure is defined as a force acting over an area. Here, the force from the expanding gases is distributed over the area of the base of the piston. In other words, the force is pressure times area.
Thus, work, which is force times distance, can be rewritten as pressure multiplied by the area and distance over which it acts. For the engine cylinder, this is the height difference the piston moves.
Considering that area times height is the volume of a cylinder, the equation for work can be defined as pressure times the volume change of the system.
However, because the volume increases and the piston is pushed downward, the system actually performs work on the surroundings, which by convention is a negative value.
Therefore, work is defined as the negative of the pressure, P, times delta-V or the change in final and initial volume, which happened during the expansion.
The unit for pressure-volume work is usually defined as liter-atmosphere. This unit can be converted to the conventional unit of energy, Joules, by using the conversion factor one liter-atmosphere is equal to 101.3 Joules.
6.5:
Quantifying Work
As a system undergoes a change, its internal energy can change, and energy can be transferred from the system to the surroundings, or from the surroundings to the system.
Energy transfer occurs through heat and work. The relationship between internal energy, heat, and work is represented by the equation:
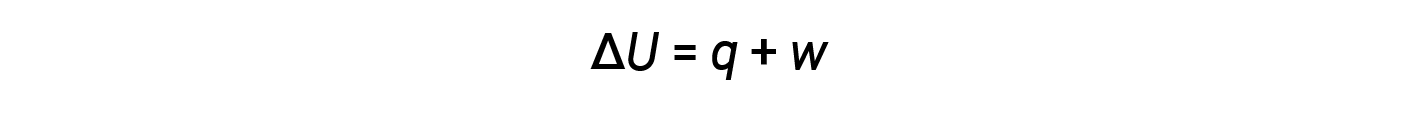
While heat is a function of an observed temperature change, work is a function of an observed volume change called the pressure-volume work. Work (w) can be defined as a force (F) acting through a distance (D).
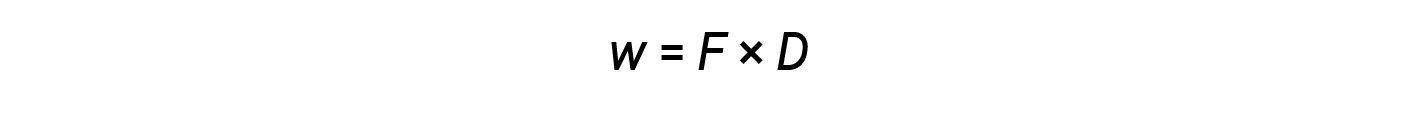
Pressure-volume work (or expansion work) occurs when a system pushes back the surroundings against a restraining pressure, or when the surroundings compress the system. An example of this occurs during the operation of an internal combustion engine. The combustion reaction of gasoline and oxygen is exothermic. Some of this energy is given off as heat, and some is performed as work by expanding the gases in the cylinder, thereby pushing the piston outward. The substances involved in the reaction are the system, and the engine and the rest of the universe are the surroundings. The system loses energy by both heating and doing work on the surroundings, and its internal energy decreases.
When the volume of a cylinder increases (i.e., the gas expands), it pushes against an external force, which is the pressure defined as force per unit area.
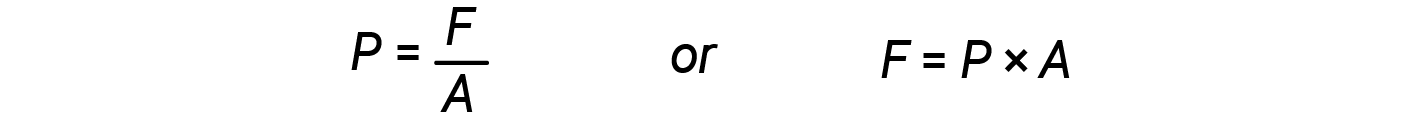
From equations 2 and 3:
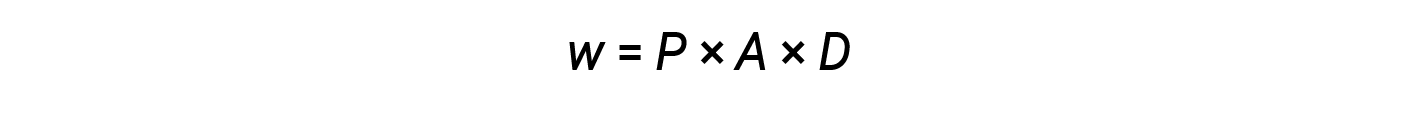
The product of area and distance (A × D) is equal to the change in the volume (ΔV) of the gas in the cylinder.
Therefore,

Since the volume increases during expansion, Vfinal > Vinitial, and ΔV is positive. However, for a positive expansion (i.e., when the system does work on the surroundings), w should be negative, and therefore, a negative sign is added to the equation.
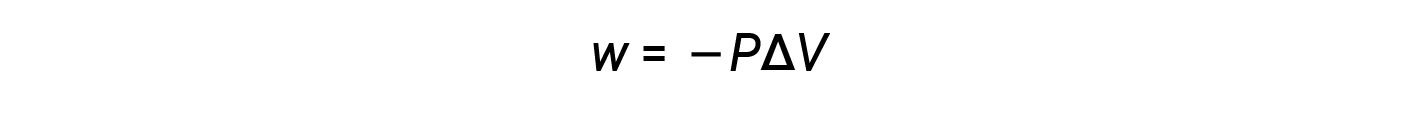
According to this equation, pressure-volume work is the negative of the external pressure (or opposing pressure) multiplied by the change in volume.
The unit of work based on this equation is L·atm. Some other useful conversions factors are:
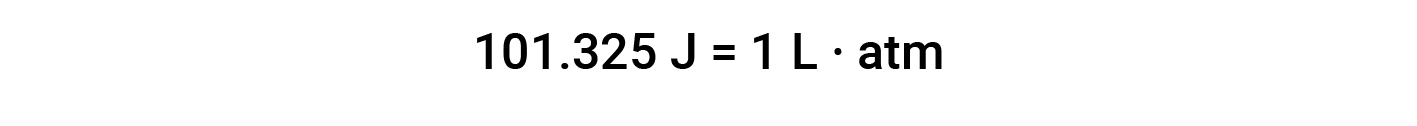
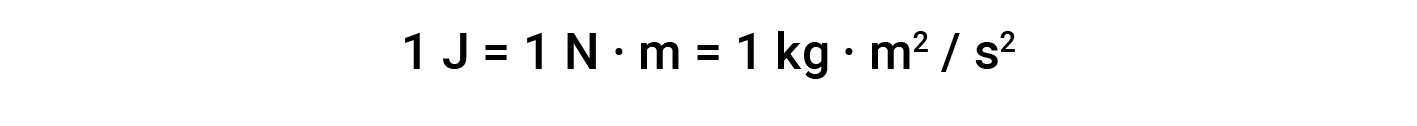
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Suggested Reading
- Schmidt-Rohr, Klaus. "Expansion work without the external pressure and thermodynamics in terms of quasistatic irreversible processes." Journal of Chemical Education 91, no. 3 (2014): 402-409.
- Gislason, Eric A., and Norman C. Craig. "General definitions of work and heat in thermodynamic processes." Journal of Chemical Education 64, no. 8 (1987): 660.
- O'Loane, J. Kenneth. "Adiabatic changes: Reversible and irreversible changes involving only pressure-volume work." Journal of Chemical Education 30, no. 4 (1953): 190.
