7.13:
The Energies of Atomic Orbitals
Atomic orbitals have different energies, as rationalized by Coulomb interactions, the shielding effect, and orbital penetration.
Coulomb’s law indicates that the attractive or repulsive force between two charged particles has an inverse-square relationship with the distance between them.
Atomic orbital sizes increase with shell number, and electrons are repelled from the space occupied by lower-shell orbitals. Thus, Coulomb's law suggests that as the shell number increases, the electrons experience less attraction to the nucleus, which corresponds to higher orbital energies.
In addition, electrons that are at about the same distance from or closer to the nucleus have a shielding effect that further reduces the attraction to the nucleus. The greater the shielding, the less attraction to the nucleus is felt. This is one reason for the differences in orbital energies within electron shells. For instance, 3s and 3p electrons significantly shield 3d electrons.
The effective nuclear charge felt by an electron is calculated by subtracting the shielding constant S, which depends on the number of shielding electrons and the subshells they occupy, from the atomic number.
For example, the two 1s electrons in lithium, which has an atomic number of three, screen its 2s electron. The shielding constant for that electron is determined from semi-empirical rules to be 1.7. Hence, the effective nuclear charge felt by the 2s electron is 1.3.
The shapes of orbitals also dictate their energy. If the electrons in an outer orbital can move far into areas occupied by inner electrons to be close to the nucleus, they will be much less shielded there. Thus, the energy of that outer orbital is lower.
This can be visualized with a radial distribution function describing the probability of finding an electron at a given distance from the nucleus.
Radial distribution function plots for the 1s, 2s, and 2p subshells reveal that 2s electrons have a modest probability of being near the nucleus, whereas 2p electrons mostly stay outside or at the outer edge of the 1s region. The 2s orbital, therefore, is said to have greater penetrating ability. In the third shell, the 3s electrons penetrate the most and the 3d electrons penetrate the least.
Generally, atomic orbital energy increases with shell number and, on the subshell level, from s to f. However, the penetration effect becomes so significant in the fourth and fifth shells that the 4s and 5s orbitals frequently have lower relative energies than the 3d and 4d orbitals, respectively.
7.13:
The Energies of Atomic Orbitals
In an atom, the negatively charged electrons are attracted to the positively charged nucleus. In a multielectron atom, electron-electron repulsions are also observed. The attractive and repulsive forces are dependent on the distance between the particles, as well as the sign and magnitude of the charges on the individual particles. When the charges on the particles are opposite, they attract each other. If both particles have the same charge, they repel each other.
As the magnitude of the charges increases, the magnitude of force increases. However, when the separation of charges is more, the forces decrease. Thus, the force of attraction between an electron and its nucleus is directly proportional to the distance between them. If the electron is closer to the nucleus, it is bound more tightly to the nucleus; therefore, the electrons in the different shells (at different distances) have different energies.
For atoms with multiple energy levels, the inner electrons partially shield the outer electrons from the pull of the nucleus, due to electron-electron repulsions. Core electrons shield electrons in outer shells, while electrons in the same valence shell do not block the nuclear attraction experienced by each other as efficiently. This can be explained with the concept of effective nuclear charge, Zeff. This is the pull exerted on a specific electron by the nucleus, taking into account any electron-electron repulsions. For hydrogen, there is only one electron, and so the nuclear charge (Z) and the effective nuclear charge (Zeff) are equal. For all other atoms, the inner electrons partially shield the outer electrons from the pull of the nucleus, and thus:

Orbital penetration describes the ability of an electron to be closer to the nucleus. The electrons in the s-orbital can get closer to the nucleus and have a more penetrating ability. The probability density for a spherical s-orbital is non-zero at the nucleus. Different subshells have different spatial orientations. Due to the dumbbell-shaped orbital, the p-electron penetrates much less. Its wavefunction has a node passing through the nucleus, where the probability of finding the electron is zero. Thus, an s orbital electron is bound more tightly to the nucleus and has lower energy than the p-electron. A d-electron has even lower penetration and higher energy than a p orbital electron.
For various shells and subshells, the trend of penetrating power of an electron can be depicted as follows
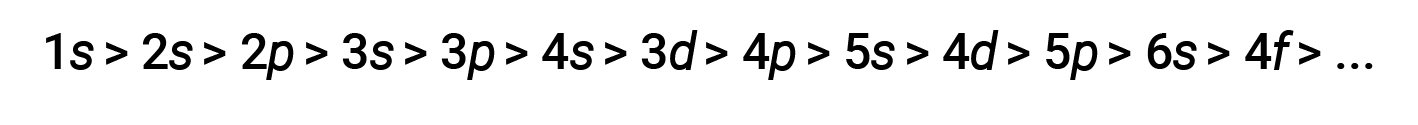
The effect of shielding and penetration is large, and a 4s electron may have lower energy than a 3d electron.
This text is adapted from Openstax, Chemistry 2e, Section 6.4: Electronic Structure of Atoms (Electron Configurations).
