7.14:
The Aufbau Principle and Hund’s Rule
Recall that atomic orbitals have different energies and can accommodate two electrons each. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and Hund’s rule of maximum multiplicity explains the filling of orbitals in the subshells.
The aufbau principle states that in the ground state, electrons fill atomic orbitals from lowest to highest energy to achieve the lowest-energy configuration.
Though energy generally increases with shell number, the greater penetration of s orbitals often leads to the four-s and five-s orbitals having lower energies than the three-d and four-d orbitals, respectively. The order can be remembered using diagrams like this one, where the path of the arrow reveals the sequence in which electrons are assigned to orbitals.
Consider writing the electron configuration for carbon — an element with atomic number six. Certainly, the 1s orbital, which has the lowest energy, should be filled before the 2s orbital. Each orbital can hold a maximum of two electrons.
The fifth electron enters a 2p subshell. But which of the three 2p orbitals does it populate?
Well! The orbitals in any subshell are presumed to be degenerate, which means that they have the same energy. Thus, the fifth electron can enter any of the three degenerate 2p orbitals.
What about the sixth electron? Does it enter the 2p orbital with an electron or one of the vacant 2p orbitals?
According to Hund’s rule of maximum multiplicity, electrons singly occupy all orbitals of a given energy level before they start pairing, and the unpaired electrons cannot have opposite spins: their spins must be parallel.
Hence, for carbon, the two 2p electrons must occupy two different orbitals and have parallel spins. This way, electrons can spread over a larger area. This decreases their shielding of each other, thereby minimizing the energy of the atom.
For nitrogen, each of the three 2p orbitals is singly occupied.
For oxygen, once the degenerate 2p orbitals are filled singly, the last electron must pair with another 2p electron. The atom has two unpaired electrons.
The electron configuration of neon reveals that the outermost shell is filled to its maximum capacity of eight electrons. Neon has two electrons, called core electrons in the inner shell, and eight electrons, called valence electrons, in the outermost shell.
7.14:
The Aufbau Principle and Hund’s Rule
To determine the electron configuration for any particular atom, we can build the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the elements. This procedure is called the aufbau principle, from the German word aufbau (“to build up”). Each added electron occupies the subshell of lowest energy available, subject to the limitations imposed by the allowed quantum numbers according to the Pauli exclusion principle. Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Figure 1 illustrates the traditional way to remember the filling order for atomic orbitals.
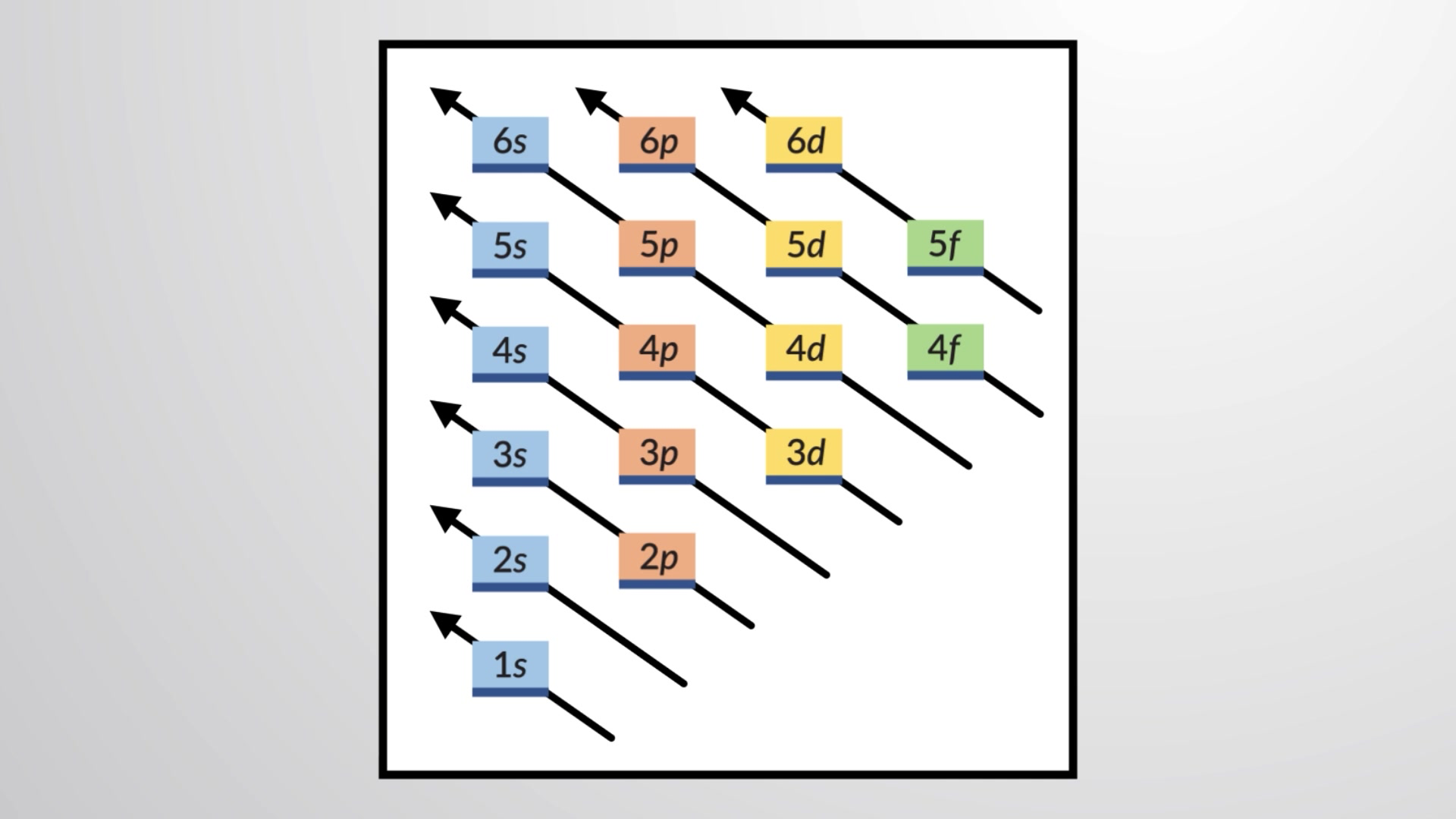
Figure 1 This diagram depicts the energy order for atomic orbitals and is useful for deriving ground-state electron configurations.
Consider writing the electron configuration for carbon—an element with atomic number six. Four electrons fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. We now have a choice of filling one of the 2p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but degenerate, p orbitals. The orbitals are filled as described by Hund’s rule: the lowest-energy configuration for an atom with electrons within a set of degenerate orbitals is that having the maximum number of unpaired electrons. Thus, the two electrons in the carbon 2p orbitals have identical n, l, and ms quantum numbers and differ in their ml quantum number (in accord with the Pauli exclusion principle). The orbital diagram for carbon, with an electron configuration of 1s22s21p2 is:
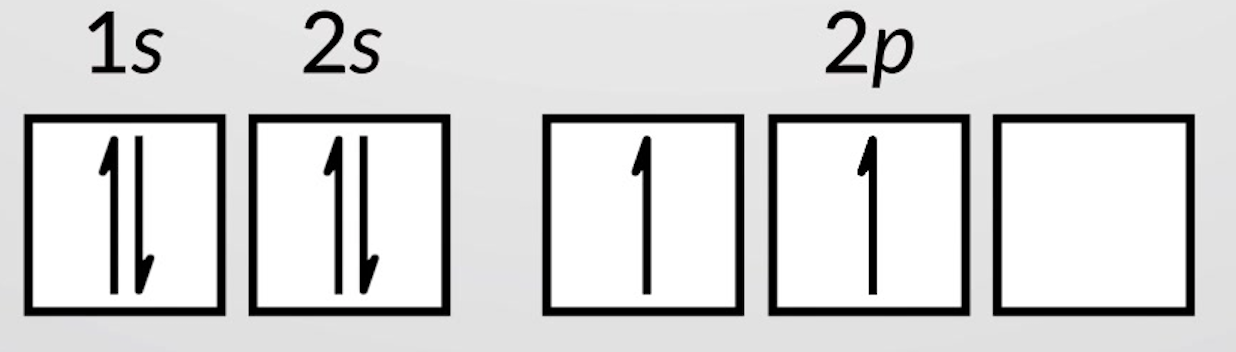
Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only one 2p orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled.
This text is adapted from Openstax, Chemistry 2e, Section 6.4: Electronic Structure of Atoms.
