12.7:
Expressing Solution Concentration
If a small amount of solute is added to a large quantity of solvent, a dilute solution is formed. The concentrations of very dilute solutions, such as trace metal impurities in water, are often expressed in parts per billion or ppb, or in parts per million or ppm.
Ppm of a solute can be calculated by dividing the mass of solute by the mass of solution and multiplying times one million.
If a large amount of solute is added to a small quantity of solvent, a concentrated solution is formed.
Commonly used units include relative units expressed as percentages, such as: mass percent, which is the mass of solute divided by the mass of solution times one hundred; volume percent, which is the volume of solute divided by the volume of solution times one hundred; and mass per volume percent, which is the mass of solute divided by the volume of solution times one hundred.
In the laboratory, these units are used along with mole fraction, molarity, and molality.
A mole fraction of component i, which can be either solute or solvent, is the number of moles of the component divided by the total number of moles of all components in the solution. This is a ratio with no units, denoted by the letter χi.
Molarity is the number of moles of solute in 1 liter of solution. Its symbol is uppercase M and the units are moles per liter.
Molality is the number of moles of solute in 1 kilogram of solvent. Its symbol is a lowercase italicized m and the units are moles per kilogram.
In molality, the mass of the solvent is considered, whereas, in molarity, the volume of the entire solution is taken into account.
To prepare a 1 molar or 1 molal solution, 1 mole of a solute, such as iodine, is dissolved in a solvent, such as carbon tetrachloride.
The final volume of the solution is made up to 1 liter to form a 1 molar solution.
For a molal solution, 1 kilogram of carbon tetrachloride is weighed out. Given that the density of carbon tetrachloride is 1.59 grams per milliliter, the volume of 1 kilogram of solvent would be 629 milliliters.
So the final concentration of 1 molal solution in carbon tetrachloride would be equal to 1.59 molar.
12.7:
Expressing Solution Concentration
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration).
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Molarity (M) is a useful concentration unit for many applications in chemistry. Molarity is defined as the amount of solute in number of moles divided by the volume of the solution in liters:
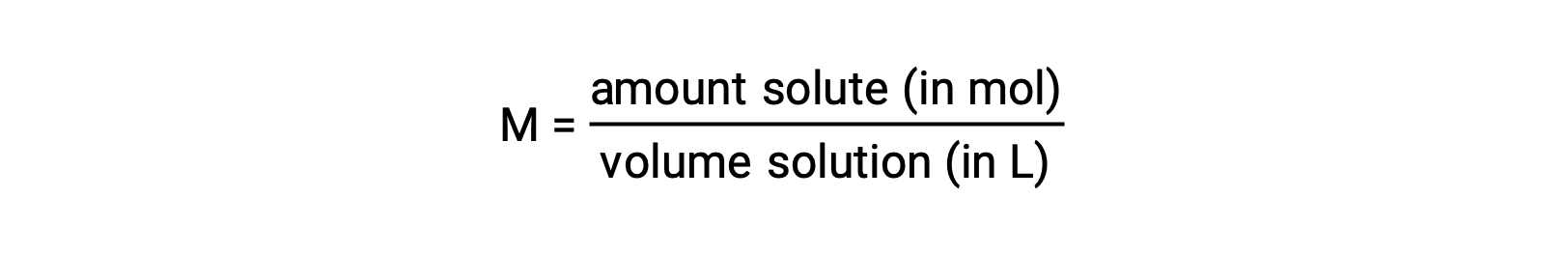
Because solution volumes vary with temperature, molar concentrations will likewise vary. When expressed as molarity, the concentration of a solution with identical numbers of solute and solvent species will be different at different temperatures due to the contraction/expansion of the solution. More appropriate for calculations involving many colligative properties are mole-based concentration units whose values are not dependent on temperature. Two such units are mole fraction (introduced in the previous chapter on gases) and molality.
The mole fraction, χA, of a component is the ratio of its molar amount to the total number of moles of all solution components:
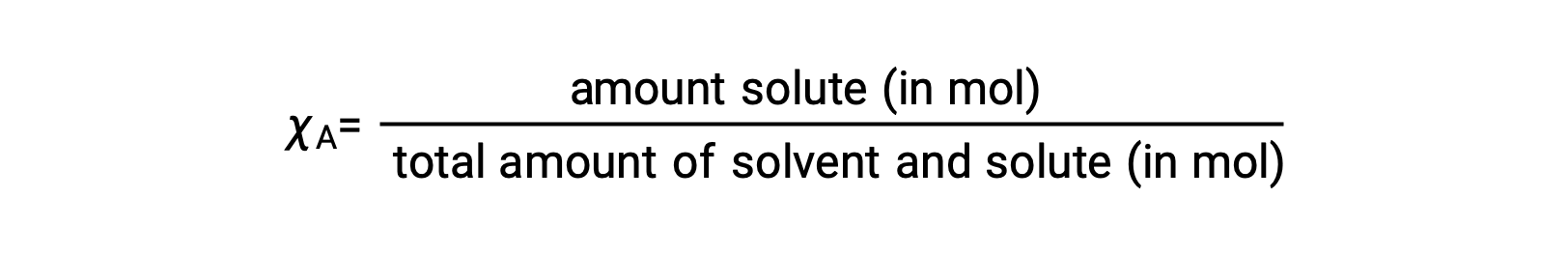
By this definition, the sum of mole fractions for all solution components (the solvent and all solutes) is equal to one.
Molality is a concentration unit defined as the ratio of the numbers of moles of solute to the mass of the solvent in kilograms:
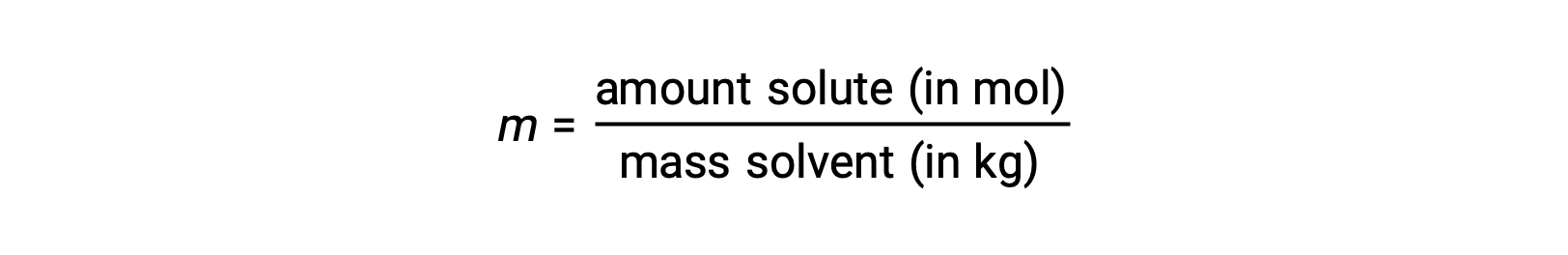
Since these units are computed using only masses and molar amounts, they do not vary with temperature and, thus, are better suited for applications requiring temperature-independent concentrations.
This text is adapted from Openstax, Chemistry 2e, Section 11.4: Colligative Properties.
Suggested Reading
- Irving, Harry MNH, Henry Freiser, and Thomas Summers West. Compendium of analytical nomenclature: definitive rules 1977. Elsevier, 2017.
