12.6:
Physical Properties Affecting Solubility
The solubility of a solute is the maximum amount that will dissolve in a given quantity of solvent at a specific temperature. This means that, by definition, temperature affects the solubility of most substances.
For most solid solutes, their solubility in water increases as the solution temperature increases, although there are exceptions, such as cesium sulfate.
As the temperature changes, the solubility of different substances increases at different rates. For instance, the solubility of potassium nitrate rises sharply with temperature, while that of potassium chloride changes very little.
When a solution containing a mixture of potassium nitrate and potassium chloride is heated and then cooled down slowly to ten degrees, the crystalline precipitate will contain more of the potassium nitrate since it is less soluble at low temperatures.
This separation technique is known as fractional crystallization.
In contrast to solid solutes, the solubility of gases in water decreases with increasing temperature.
If a beaker of cold soda is warmed to room temperature, the soda flattens quickly. This is because the solubility of carbon dioxide decreases with increasing temperature.
The solubility of gases also depends on pressure. The higher the pressure of a gas above a liquid, the more soluble the gas is in the liquid.
This relationship between pressure and the solubility of a gas is quantified by Henry’s Law, which states that the solubility of a gas equals the Henry's Law Constant multiplied by the pressure.
While making soda, carbon dioxide is dissolved in a sugar solution under high pressure. The pressure above allows the solution to become saturated with carbon dioxide.
Thus, when a soda can is opened, we can hear the familiar pop as the pressure is released and see many bubbles form as the carbon dioxide gas escapes from the solution.
12.6:
Physical Properties Affecting Solubility
Solutions of Gases in Liquids
As for any solution, the solubility of a gas in a liquid is affected by the attractive intermolecular forces between solute and solvent species. Unlike solid and liquid solutes, however, there is no solute-solute intermolecular attraction to overcome when a gaseous solute dissolves in a liquid solvent since the atoms or molecules comprising a gas are far separated and experience negligible interactions. Consequently, solute-solvent interactions are the sole energetic factor affecting solubility. For example, the water solubility of oxygen is approximately three times greater than that of helium (there are greater dispersion forces between water and the larger oxygen molecules) but 100 times less than the solubility of chloromethane, CHCl3 (polar chloromethane molecules experience dipole-dipole attraction to polar water molecules). Likewise, note the solubility of oxygen in hexane, C6H14, is approximately 20 times greater than it is in water because greater dispersion forces exist between oxygen and the larger hexane molecules.
Temperature is another factor affecting solubility, with gas solubility typically decreasing as temperature increases. This inverse relation between temperature and dissolved gas concentration is responsible for one of the major impacts of thermal pollution in natural waters.
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Gas solubility increases as the pressure of the gas increases.
For many gaseous solutes, the relation between solubility, Sgas, and partial pressure, Pgas, is a proportional one:
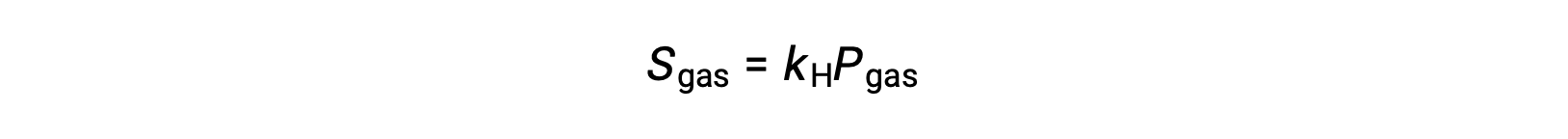
where kH is a proportionality constant that depends on the identities of the gaseous solute and solvent and on the solution temperature. This is a mathematical statement of Henry’s law: The quantity of an ideal gas that dissolves in a definite volume of liquid is directly proportional to the pressure of the gas.
This text is adapted from Openstax, Chemistry 2e, Section 11.3: Solubility.
Suggested Reading
- Kolev, Nikolay Ivanov. "Solubility of O 2, N 2, H 2 and CO 2 in water." In Multiphase Flow Dynamics 4, pp. 209-239. Springer, Berlin, Heidelberg, 2011.
- Mitchell, Alan J., ed. Formulation and production carbonated soft drinks. Springer Science & Business Media, 1990.
