12.7:
溶液濃度の表現
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Expressing Solution Concentration
If a small amount of solute is added to a large quantity of solvent, a dilute solution is formed. The concentrations of very dilute solutions, such as trace metal impurities in water, are often expressed in parts per billion or ppb, or in parts per million or ppm. Ppm of a solute can be calculated by dividing the mass of solute by the mass of solution and multiplying times one million. If a large amount of solute is added to a small quantity of solvent, a concentrated solution is formed. Commonly used units include relative units expressed as percentages, such as: mass percent, which is the mass of solute divided by the mass of solution times one hundred; volume percent, which is the volume of solute divided by the volume of solution times one hundred; and mass per volume percent, which is the mass of solute divided by the volume of solution times one hundred. In the laboratory, these units are used along with mole fraction, molarity, and molality. A mole fraction of component i, which can be either solute or solvent, is the number of moles of the component divided by the total number of moles of all components in the solution. This is a ratio with no units, denoted by the letter χi. Molarity is the number of moles of solute in 1 liter of solution. Its symbol is uppercase M and the units are moles per liter. Molality is the number of moles of solute in 1 kilogram of solvent. Its symbol is a lowercase italicized m and the units are moles per kilogram. In molality, the mass of the solvent is considered, whereas, in molarity, the volume of the entire solution is taken into account. To prepare a 1 molar or 1 molal solution, 1 mole of a solute, such as iodine, is dissolved in a solvent, such as carbon tetrachloride. The final volume of the solution is made up to 1 liter to form a 1 molar solution. For a molal solution, 1 kilogram of carbon tetrachloride is weighed out. Given that the density of carbon tetrachloride is 1.59 grams per milliliter, the volume of 1 kilogram of solvent would be 629 milliliters. So the final concentration of 1 molal solution in carbon tetrachloride would be equal to 1.59 molar.
12.7:
溶液濃度の表現
溶質とは、溶液中に含まれる成分のうち、通常は溶媒よりもはるかに低い濃度で存在するものを指します。溶質の濃度は、希薄(比較的低濃度)や濃厚(比較的高濃度)といった定性的な言葉で表現されることが多いです。
濃度を定量的に評価するには、用途に応じてさまざまな測定単位を用いることができます。モル濃度(M)は、化学の多くの応用に役立つ濃度単位です。モル濃度は、溶質の量(モル数)を溶液の体積(リットル)で割ったものと定義されています。
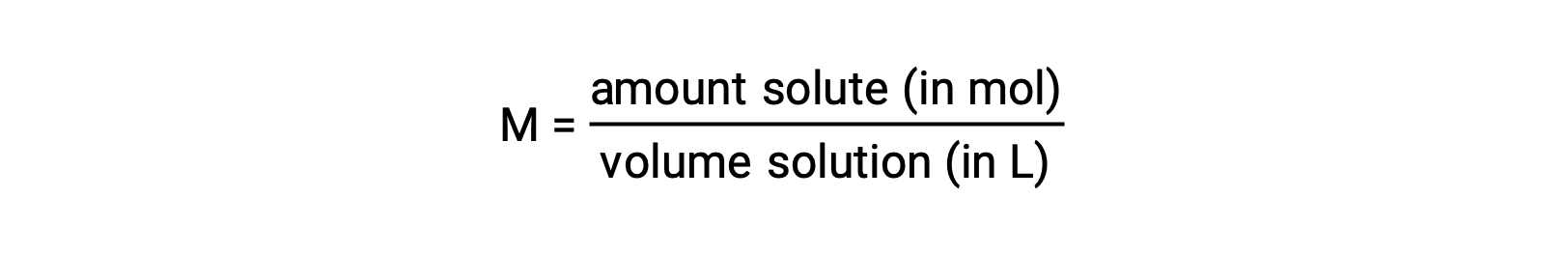
溶液の体積は温度によって変化するため、モル濃度も同様に変化します。モル濃度で表すと、溶質種と溶媒種の数が同じである溶液の濃度は、溶液の収縮・膨張により、温度が異なると異なる値になります。多くの束一的性質を含む計算には、値が温度に依存しないモルベースの濃度単位がより適しています。そのような単位として、モル分率(前章の気体の章で紹介した)とモル濃度があります。
モル分率とは、溶液の全成分のモル数に対する、その成分のモル量の割合です。<span style="color: rgb(77, 81, 86); font-family:
χA
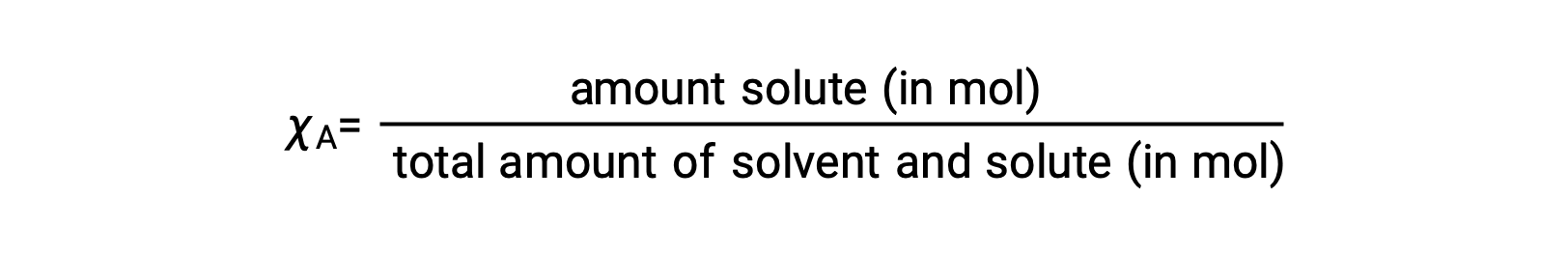
この定義では、すべての溶液成分(溶媒とすべての溶質)のモル分率の合計は1になります。
モル濃度とは、溶媒の質量(キログラム)に対する溶質のモル数の比として定義される濃度の単位です。
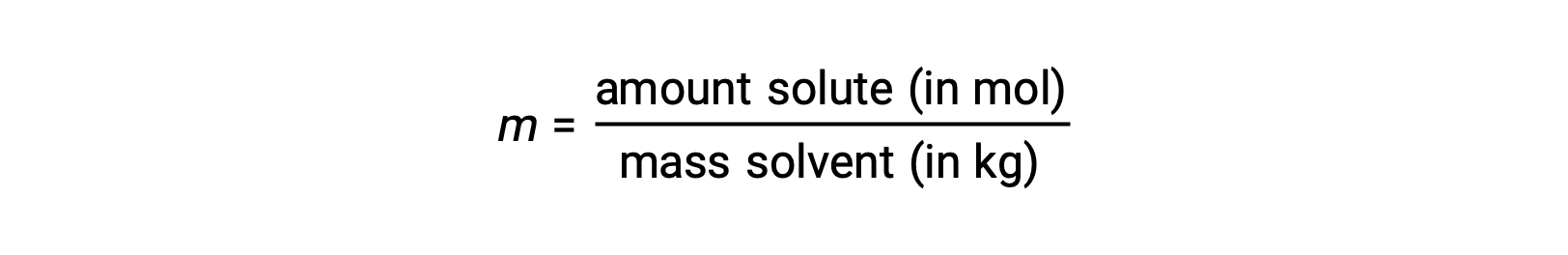
これらの単位は、質量とモル量のみで計算されるため、温度による変化がなく、温度に依存しない濃度を必要とする用途に適しています。
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Section 11.4: Colligative Properties.
Suggested Reading
- Irving, Harry MNH, Henry Freiser, and Thomas Summers West. Compendium of analytical nomenclature: definitive rules 1977. Elsevier, 2017.
