14.3:
気相反応及び不均一系反応の平衡
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Core
Chemistry
Homogeneous Equilibria for Gaseous Reactions
For chemical reactions, where the reactants and products are all gases, the equilibrium constant can also be calculated using the individual partial pressures rather than their molar concentrations. Thus, when gases A and B convert to gases C and D in a reversible reaction, the equilibrium expression can be written instead as the partial pressure of each gas, raised to their stoichiometric coefficients. The equilibrium constant is designated as Kp, where the subscript p indicates pressure. For a given gaseous reaction, Kp is not necessarily equal to Kc, because the partial pressure of a gas and its molar concentration are separate values. However, a relationship can be derived between the two constants using the ideal gas equation and the definition of molarity. To derive this relationship, consider the equilibrium expressions for Kc and Kp for the given chemical reaction. The ideal gas equation relates the pressure of a gas to its number of moles and its volume at a given temperature. Substituting the ratio of moles to volume for molarity in the ideal gas equation allows the pressure of an ideal gas to be expressed in terms of its molar concentration. In this way, the individual partial pressures in the expression for Kp can be substituted for the concentration equivalent of each gas. The stoichiometric coefficients remain unchanged. In the modified expression of Kp, the ratio of the concentration of the products to the concentration of reactants can be substituted for Kc. This equation gives the relationship between the two constants — Kp equals Kc times RT raised to the sum of the coefficients of the products minus the sum of the coefficients of the reactants. The difference between the coefficients of gaseous reactants and products can be represented as Δn.
14.3:
気相反応及び不均一系反応の平衡
気相反応における均一系平衡
気相反応の場合、平衡定数は、反応物と生成物のモル濃度(Kc)または分圧(Kp)のいずれかで表すことができます。これら2つのK値の関係は、理想気体の方程式とモル度の定義から簡単に導き出すことができます。理想気体の方程式によると、次が成立します。
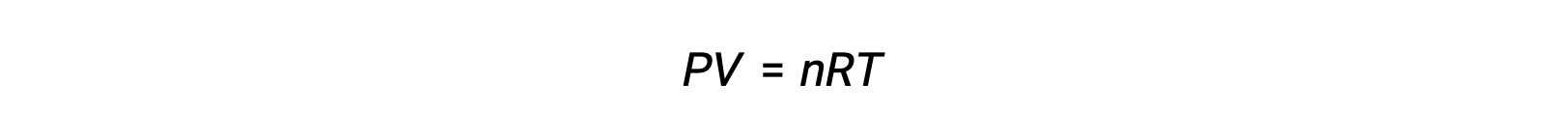
モル濃度または、モル数を体積で割って求められます。

そのため、次が成立します。
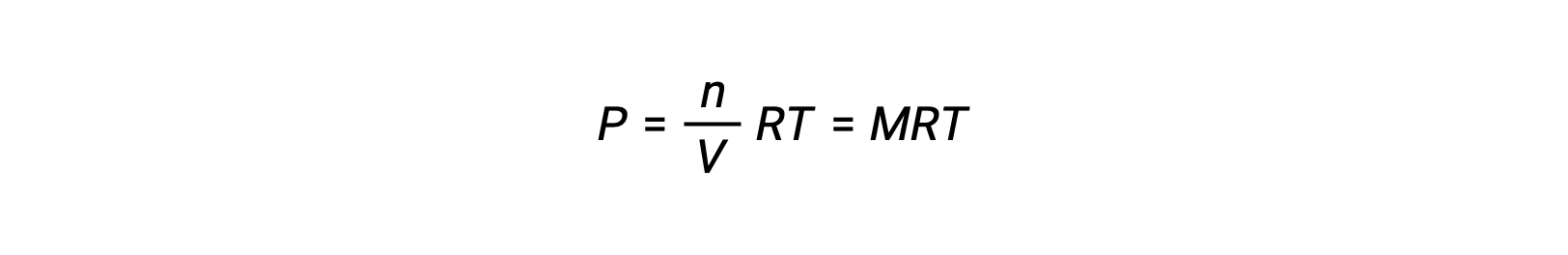
ここで、Pは分圧、Vは体積、nはモル数、Rは気体定数、Tは温度、Mはモル濃度です。
気相反応の場合 m A + n B ⇌ x C + y D です。
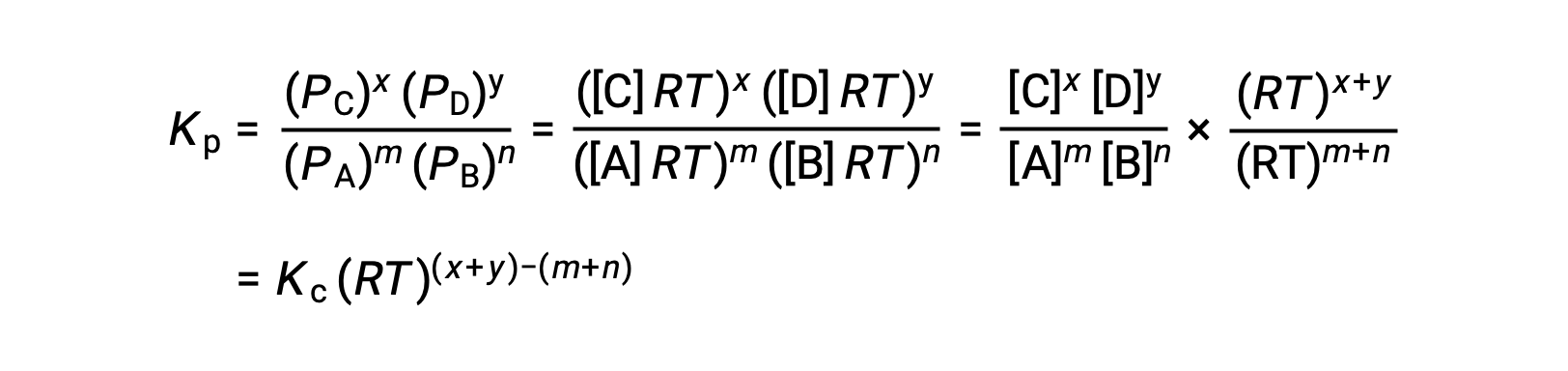
そのため、KcとKPの関係は、次の通りです。
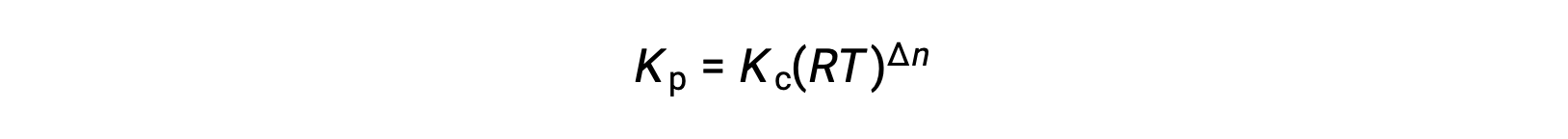
ここで Δn は、この場合、生成物と反応物の気体のモル量の差です。
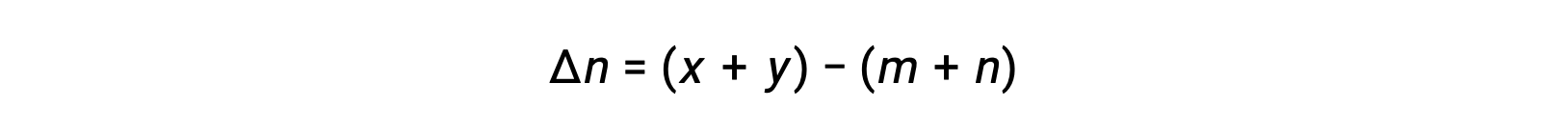
上記の文章は以下から引用しました。 Openstax, Chemistry 2e, Section 13.2 Equilibrium Constants.
