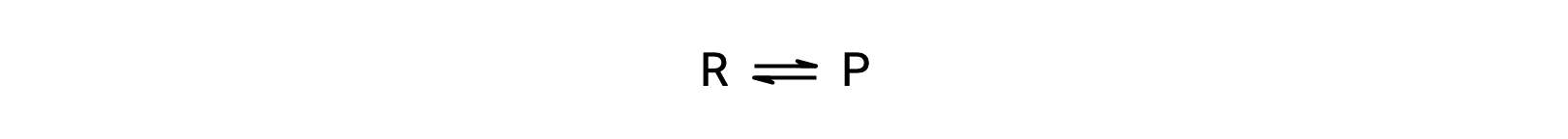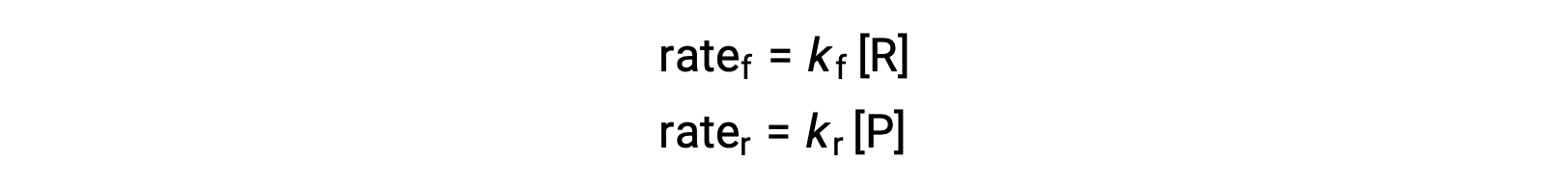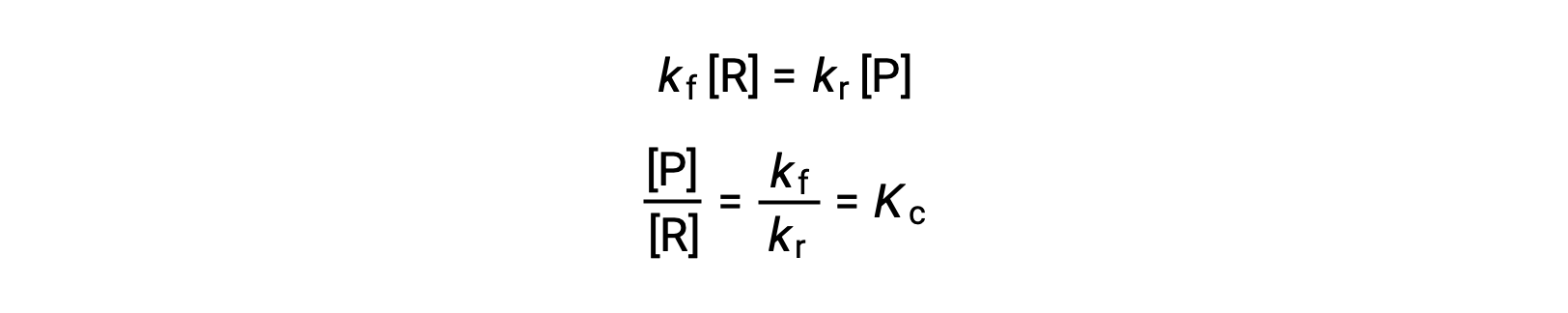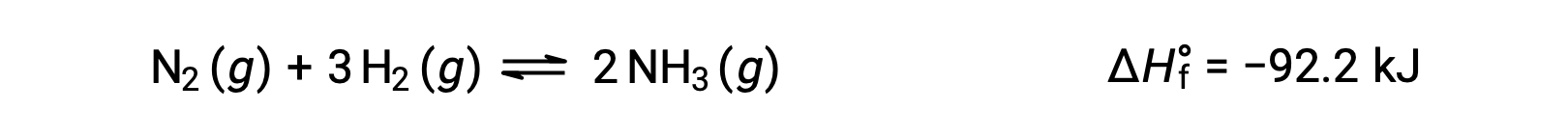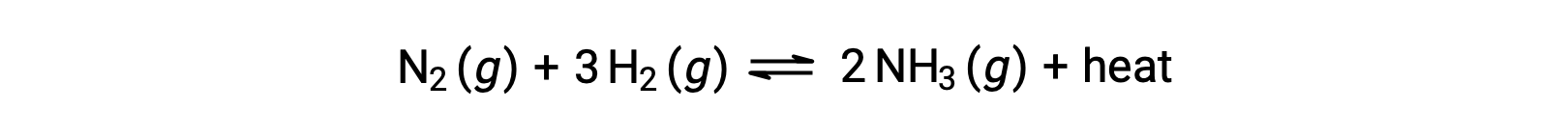Temperature affects the rate of a chemical reaction; therefore, a change in temperature for a reaction at equilibrium acts as a stress on the system. Le Châtelier's principle predicts how the system will respond to minimize such disturbances. A change in the temperature changes the value of the equilibrium constant, unlike a change in concentration or volume, which shifts the equilibrium without changing the value of K. Consider the decomposition of gaseous phosphorus pentachloride into phosphorus trichloride and chlorine gas. For this endothermic reaction, the heat absorbed can be thought of as a reactant. An increase in temperature adds heat to the system, similar to adding more of a reactant. Thus, the equilibrium position shifts towards the products and produces more phosphorus trichloride and chlorine to consume the extra heat because the value of the equilibrium constant, K, has increased. On the other hand, a decrease in temperature removes heat from the system, similar to removing a reactant. The equilibrium position shifts towards the reactants and produces more phosphorus pentachloride to release heat because the value of K has decreased. For an exothermic reaction, such as the gaseous reaction between sulfur dioxide and oxygen to produce sulfur trioxide, the heat released can be thought of as a product. An increase in temperature is similar to adding more of a product. This causes the equilibrium position to shift toward the reactants, producing more sulfur dioxide and oxygen to absorb some of the added heat because the value of K has decreased. Conversely, decreasing the temperature of this exothermic reaction removes heat, like removing a product. The equilibrium position shifts towards the products and produces more sulfur trioxide to release heat as K has increased. Thus, an increase in temperature favors the products in an endothermic reaction, whereas a decrease in temperature favors the products in an exothermic reaction.